Background
Morphine was discovered in early 1800 by 21-year old Freidrich Wilhelm Serturner. Through curiosity, Serturner got interested in the opium medicinal plant which was used by 18th-century physicians. His main concern was the medicinal properties of opium (Clark & Graham, 2008). He however used his free time studying the plant and in 1805, and he isolated an organic alkaloid compound from the resinous gum which is secreted by papaver somniferum, referred to as the opium poppy. Further series of experiments demonstrated that opium with the alkaloid did not affect animals. However, the alkaloid effect was ten times stronger than processed opium. Serturner named the alkaloid morphine after the Greek god of dreams (morpheus), for it caused sleep. In the following years, he learned its therapeutic effects as well as its substantial dangers. Morphine was the first isolated substance of a natural alkaloid. It led to considerable interest and research of alkaloid chemistry and accelerated the emergence of the modern pharmaceutical industry. Later research reported that morphine was the potential for addiction. However, by 1850, morphine had already hastened as the key method of reducing pain during and after surgery (Hammer & Golianu, 2007).
Introduction
The two groups of alkaloids present in opium are phenanthrenes and isoquinolines. Morphine is categorized as phenanthrene together with codeine and thebaine. Morphine has shown a major significant effect on the central nervous system (CNS). Morphine is the most prevalent and commonly used alkaloid in opium. However, many side effects have been associated with morphine such as respiratory difficulties, coma, lung edema, cardiac arrest, and many more (Gullo & Pezzilli, 2000). The normal lethal dose for morphine has been approximated as about 120 and above milligrams. After administration in the body, morphine binds to u-opioid receptors in the spinal cord, brain, and intestine. Clinical researches carried out in several laboratories have confirmed the presence of morphine in human and animal tissue. The chemical structure of morphine is illustrated below;
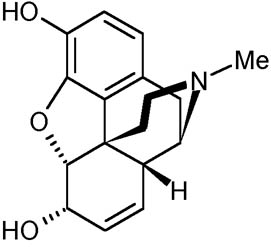
Clinical pharmacology
Various researchers have confirmed that morphine is a pure opioid agonist which is relatively selective for the mu receptor. However, it may also interact with other opioid receptors at higher doses. In addition to analgesia, the widely diverse effects of morphine include drowsiness, changes in mood, respiratory depression, decreased gastrointestinal motility, nausea, vomiting, and alterations of the endocrine and autonomic nervous system.
Effects on the Central Nervous System
Morphine largely affect the central nervous system (CNS) since its principal therapeutic action is analgesia. Other therapeutic effects of morphine include anxiolysis, euphoria and feelings of relaxation. Although the precise mechanism of the analgesic action is unknown, specific CNS opiate receptors and endogenous compounds with morphine-like activity have been identified throughout the brain and spinal cord and are likely to play a role in the expression and perception of analgesic effects. In common with other opioids, morphine causes respiratory depression, in part by a direct effect on the brainstem respiratory centers. In addition, morphine and other related opioids depress the cough reflex by a direct effect on the cough center in the medulla. With lower doses than those required for analgesia, antitussive effects may occur. Further, morphine has been linked with miosis disorder even in total darkness. Pinpoint pupils are a sign of opioid overdose; however, when asphyxia is present during opioid overdose, marked mydriasis occurs.
Effects on the Gastrointestinal Tract
Gastribiliary and pancreatic secretions are usually decreased by morphine. Morphine causes a reduction in motility and is associated with an increase in tone in the antrum of the stomach and duodenum. This leads to a delay in food digestion in the small intestines and decreased propulsive contractions. Propulsive peristaltic waves in the colon are decreased, while the tone is increased to the point of spasm resulting in severe constipation. Moreover, a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi and spasms of the sphincter of the urinary bladder is caused by high intake of morphine.
Effects on the Cardiovascular System
In therapeutic doses, morphine does not usually exert major effects on the cardiovascular system. Morphine produces peripheral vasodilation which may result in orthostatic hypotension and fainting. Release of histamine can occur, which may play a vital role in opioid-induced hypotension. Manifestations of peripheral vasodilation may include pruritus, flushing, red eyes as well as sweating (Skarke, et al. 2003).
Pharmacodynamics
Morphine concentrations are not predictive of analgesic response, especially in patients previously treated with opioids. The minimum effective concentration varies widely and is influenced by a variety of factors, including the extent of previous opioid use, age factor, and general medical condition. Effective doses intolerant patients may be significantly higher than in opioid-naïve patients. However, in all patients, the dose of morphine should be titrated on the basis of clinical evaluation of the patient and to achieve a balance between therapeutic and adverse effects (Walker, 2008).
Distribution
Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen and brain. Although the primary site of action is the CNS, only small quantities cross the blood-brain barrier. Morphine also crosses the placental membranes and has been reported to be present in breast milk. The volume of distribution of morphine is approximately 1 to 6 L/kg, and morphine is 20 to 35% reversibly bound to plasma proteins. However, the oral bioavailability of morphine is less than 40% and shows large inter-individual variability due to extensive pre-systemic metabolism.
Metabolism
Morphine’s major detoxification pathway is through conjugation either with D-glucuronic acid to produce glucuronides or with sulfuric acid to produce morphine-3-ethereal sulfate. A small fraction (less than 5%) of morphine is demethylated while the rest is converted by hepatic metabolism to the 3- and 6-glucuronide metabolites in the ratio of M3G:M6G, about 50% and 15%, respectively (Dinda, Gitman, & Pravin, 2005; Andersen, et al. 2002). Recent research suggested the possibility of M6G as having analgesic activity though it crosses the blood-brain barrier poorly. On the other hand, M3G has no analgesic activity. Excretion Morphine dose is mostly excreted in urine as M3G and M6G, with subsequent elimination of morphine occurring primarily as renal excretion of M3G (Skarke, Geisslinger & Lötsch, 2003). Approximately 10% of the dose is excreted as pure morphine compound in urine. Other excretion takes place in the bile where a small amount of glucuronide conjugates is excreted with minor enterohepatic recycling. Further excretion occurs in the feces where 7-10% of administered morphine is removed from the body. More research on this area has approximated the mean plasma clearance in adults as about 20 to 30 ml/min/kg and a terminal half-life of morphine after the IV administration to be 2 hours. Elderly patients (aged 65 and above) have increased sensitivity to morphine compared to other group age. In nursing mothers, low levels of morphine sulfate have been detected in breast milk (Bouwmeester, et al., 2004). The amount of morphine delivered to the infant depends on the plasma concentration of the mother, the amount of milk ingested by the infant, and the extent of first-pass metabolism. Morphine pharmacokinetics has been reported to be significantly altered in subjects with cirrhosis and renal failure (Conway, et al. 2006). The M3G and M6G to morphine plasma AUC ratios also decreased in these subjects, indicating diminished metabolic activity (Lotsch & Geisslinger, 2001).The two compounds, M3G and M6G are illustrated in figure 2;
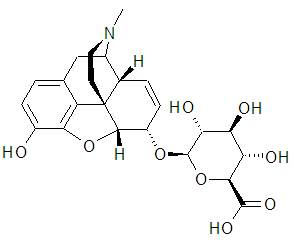
Conclusion
Morphine is an opioid agonist and a Schedule II controlled substance and is subject to criminal diversion (Bettelheim et al. 2007). Morphine may be taken by drug abusers and people with addiction disorders. For instance, drug addicts may use morphine for non-medical purposes regardless of its harmful effect on the body. Physicians and pharmacists should consider the factor of drug abuse while prescribing morphine drugs. For instance, AVINZA drugs are morphine sulfate extended-release capsules, administered daily to relieve severe pain that requires around-the-clock opioid therapy for an extended period (Nelson & Cox, 2008). When AVINZA drug is chewed or injected intravenously into the body, opioid is immediately released and this may lead to an increased amount in the body and death. Other complications that may occur include pulmonary diseases. In addition, while taking morphine-related drugs, the patient should not consume alcohol as it increases the release of morphine.
References
- Gullo, L. & Pezzilli, R. (2000). Acute pancreatitis is unlikely after morphine administration. Digestive and Liver Disease, 32(1), 74.
- Clark, D. & Graham, F. (2008). Morphine, cocaine, and 19th century cancer care. The Lancet Oncology, 9(10), 26-34.
- Dinda, A., Gitman, M. & Pravin, S. (2005). Immunomodulatory effect of morphine: therapeutic implications. Expert opinion on Drug safety, 4(4), 669-675
- Skarke, C., Geisslinger, G. & Lötsch, J. (2003). Is morphine-3-glucuronide of therapeutic relevance? Pain, 116(3), 177-180
- Bouwmeester, N. J., Anderson, B. J., Tibboel, D. et al. (2004). Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. British Journal of Anaesthesia, 92(2), 208-217.
- Hammer, G. B. & Golianu, B. (March, 2007). Opioid analgesia in neonates following cardiac surgery. Seminars in Cardiothoracic and Vascular Anesthesia, 11(1), 47–58.
- Walker, S. M. (2008). Pain in children: recent advances and ongoing challenges. British Journal of Anaesthesia, 101(1), 101-110.
- Skarke, C., Helmut S., Gerd, G. et al. (2003). Pharmacokinetics of morphine are not altered in subjects with Gilbert’s syndrome. British Journal of Clinical Pharmacology, 56(2), 228–231.
- Nelson, D. & Cox, M. (2008). Lehninger Principles of Biochemistry, (5th ed.), New York: WH Freeman and Company.
- Bettelheim et al. (2007). Introduction to general, organic, and biochemistry, Canada: Thompson Learning Inc.
- Lotsch, J. & Geisslinger, G. (2001). Morphine-6-Glucuronide: An Analgesic of the Future? Clinical Pharmacokinetics, 40(7), 485-499.
- Andersen, G., Christrup, L., Sjogren, P., et al. (2002). Changing M3G/M6G ratios and pharmacodynamics in a cancer patient during long-term morphine treatment. Journal of Pain Symptom Management, 23:161–164.
- Conway, B., Fogarty, D., Nelson, W. et al. (2006). Opiate toxicity in patients with renal failure. British Medical Journal, 332, 345-346.