Introduction
The study is based on the principles of green chemistry that can be considered as the main notions regarding the importance of the responsibility to the environment in different forms and along with the issues related to Chemistry and compositions of different organic systems. Due to the twofold importance of these components of green chemistry, it is vital to take into consideration the factors that are related to the said issues.
One component of the study in Green Chemistry is the fact that the related principles are needed to be taken into consideration. Such principles point out the responsibility that is needed to be held by the people who manufacture and develop different forms of materials and compounds specifically the kinds that can affect the organic systems or the systems of life. These issues target waste management, safe chemicals, and renewable materials production, and other related activities in chemistry that view and present the importance of the surroundings (Anastas and Warner, 1998).
In relation to the green chemistry principles, the main objective of the study is to be able to focus on the determination and presentation of possible alternate catalysts for organic reactions and synthesis that can confirm the green chemistry principles.
Prior Work
To be able to present a view and achieve an understanding of the issue under study, the review of the published research works is included. Also through the study of the said lien of works, the development and improvement of the field can be determined.
The study is mainly related to the organic compounds that are used in the chemical reactions as catalysts that can be considered to have comparatively low hazardous effects. One of the options that are being explored by different scientists and researchers is the capability of naturally occurring compounds and their components to be able to generate a catalytic effect on the system.
One example that can be cited in relation to the said compound is water. Although water can be a very stable compound, the energy that can be produced from the forces can be considered cataclysmic potential. Also, the modification compounds in relation to the reaction with water are also an important line of research since the resource can be accessed easily. Another important characteristic is the fact that water is a solvent of low reactivity. Thus, it is safer compared to other solvents (Gilbertson, Szymczak, and Tyler, 2005).
Basically, the main focus of the research is the cataclysmic action of organic compounds. One study was related to the development of new ligand frameworks. These frameworks have capabilities that can make multifunctional catalysis possible. The requirements for the catalysts are the characteristics such as modular assembly and facile electronic fine-tuning. Also, the spectroscopic signatures are also considered vital to the study (Mascarenhas, Duffey, Liu and Morken, 1999).
Proposed Work
Based on the different studies that attempt to explore clean and green chemistry, the most important reaction that can be undertaken is in the most important solvent which is water. These reactions explore the chemical agents that have a distinct reaction to water.
Aside from being a vital solvent, water also has catalytic capabilities. The objective of the study to be conducted then is to be able to locate a chemical catalyst like water in terms of catalytic effects.
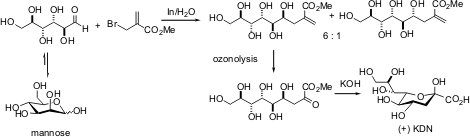
Included in the characteristics for determination are water solubility and renewable materials. These properties can be considered important on the basis of the green chemistry specifically in terms of the protection-deprotection of the sequences in the majority of compounds. “One example is with regards to the synthesis of higher carbon-sugar (+)-3-deoxy-D-glycero-D-galacto-nonulosonic acid (KDN) from carbohydrate” (Varma, 2007).
Another important characteristic of an alternative catalyst is the capability for the development of high atom efficiency catalytic processes. The said chemical characteristic can be considered essential for catalyst recycling and product isolation.
Atom efficiency catalytic process can be determined through catalyst recycling and also product isolation. The reactants and the catalysts are required to have minimal water solubility. For the reactants, the separation from water is needed to be undertaken through simple processes. In terms of the catalysts, low solubility means the ability to be reused in another round of the reaction (Wei, Keh, Li and Varma, 2005).
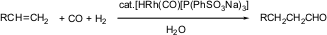
In a system with the capability to recycle the catalyst basically in the aqueous solution, pollution of the medium or the site of the reaction can be prevented even for repeated reaction. “Examples of such systems are catalytic hydrogenations, hydroformylations, the Wacker oxidation, and some polymerization reactions”. In the industries, one of the common mechanisms is the hydroformylation process that involves the catalysis of the aqueous medium. One distinctive example that can be cited is the recovery of rhodium catalyst toward the production of sulfonated phosphane ligands which are soluble in water (Varma, 2007).
“The Ruhrchemie/Rhône-Poulenc process can be recognized as optimized hydroformylation undertaken through the use of HRh(CO)(tppts)3 catalyst”. Through the use of simple phase separation, the output can be retrieved. On the other hand, the catalyst solution can be reused through a recharging reactor to allow another reaction process (Varma, 2007).
Organocatalysts
In terms of the important catalysts that can be classified in conformity to the rules of green chemistry is organocatalysis. The said process involves small organic molecules to be able to increase the rate of reaction in the system.
These molecules can be considered as the alternatives for the catalysts of organic systems that include certain examples such as C, H, O, N, S, and P. There are different reactions that can be classified based on the green chemistry principles.
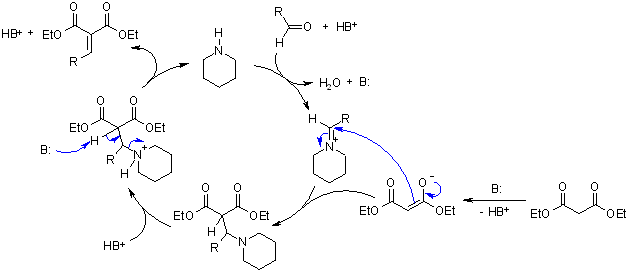
One example is the Knoevenagel Condensation; the piperidine produces an iminium ion which is a reactive intermediate prior to the production of the carbonyl compound. Due to the said mechanism, an increase of the rate of reaction can be achieved.
Another iminium production can be observed in the proline-derived compounds. These compounds are known to be strong organocatalysts that are used in carbonyl compound transformations.
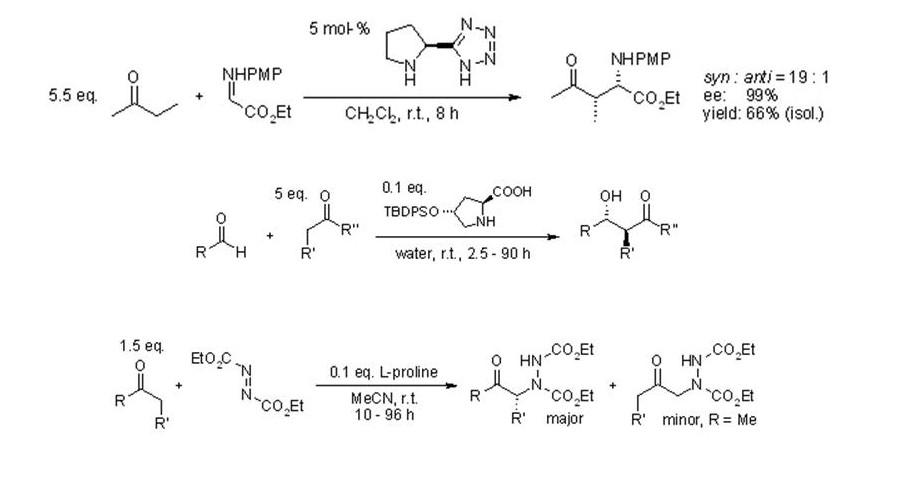
The consideration of the organocatalysts can be considered based on the advantages that they can present that can be considered to conform to the green chemistry principles. One is the lack of sensitivity to moisture and oxygen which is an indication of its nature of being easy to handle and not too reactive. The low toxicity can also be attested. Also these organocatalysts are readily available thus the use of such molecules can also be considered as cost-efficient.
Due to the said characteristic benefits, the delicate industries such as the production of pharmaceutical intermediates re largely dependent on the said chemical agents, organocatalysts are safer that reactions that involve (transition) metal catalysts.
These chemical agents are examples of the green chemistry catalysts that can be used to be able to achieve the goals of industries that can be safer for the surroundings while catalyzing the needed products.
References
- Anastas, P. and Warner, J. (1998) Green Chemistry: Theory and Practice. New York: Oxford University Press.
- Gilbertson, J. D., Szymczak, N. K. and Tyler, D. R. (2005) Reduction of N2 to Ammonia and Hydrazine Utilizing H2 as the Reductant. J. Am. Chem. Soc. 2005, 127, 10184-10185.
- Mascarenhas, C. M., Duffey, M. O., Liu, S.-Y. and Morken (1999) Simple Metal Alkoxides as Effective Catalysts for the Hetero-Aldol-Tishchenko Reaction. J. P. Org. Lett. 1999, 1, 1247-1249.
- Varma, R. S. (2007) Clean Chemical Synthesis in Water. U. S. Environmental Protection Agency
- Wei, W., Keh, C.C.K., Li, C.-J. and Varma, R.S. (2005) Water as a Reaction Medium for Clean Chemical Processes. Clean Technologies and Environmental Policy, 7, 62 (2005).
- Berkessel, A. and Gröger, H. (2005) Asymmetric Organocatalysis. Wiley VCH.
- Bonifacio, V. D. B. (2007) Proline Derivatives in Organic Synthesis. Org. Chem. Highlights 2007.