Problem Statement
This investigation aimed to apply Hess’s Law to estimate the enthalpy change of three chemical equations by calculating the heat transferred in a calorimeter. The objective was to determine the final reaction’s enthalpy change reasonably and confirm the applicability of Hess’s Law.
Hypothesis Statement
Hess’s Law is applied to show that the three reactions’ enthalpy change is independent of the route followed toward the end products. The cost of conducting an investigation is a real-world consideration that must be considered to validate Hess’s Law (Pecchi et al., 2020). In response, the investigation’s cost has been substantially minimized using software to simulate the reactions and validate the results. Additionally, the applied calculation equation for the lab is qreaction = -m * Cwater * ΔT.
Methods and Materials
The Materials and Equipment Used
- Distilled water.
- Solid NaOH.
- 0.50 M HCl solution.
- 50-mL graduated cylinders.
- Foam cups.
- A balance.
- Thermometer.
Procedure
- I accessed the lab environment by clicking the Virtual Lab link and prepared the trial materials and equipment.
- Afterward, I transferred 50.0 mL of distilled water to the solid NaOH in the foam cup through the graduated cylinder, recorded its initial temperature, and cleared the workbench.
- I tared the balance after adding the foam cup, then weighed 1.00 g of solid NaOH pellets.
- This was followed by pouring all the contents of the graduated cylinder into the foam cup and immediately recording the highest temperature observed and the mass of its contents.
- After clearing the workbench, I repeated the steps but replaced the 50 mL of water with 50 mL of the 0.50 M HCl solution. After mixing the HCl solution with the solid NaOH, I immediately recorded the highest observed temperature and cleared the workbench.
- Finally, I repeated the initial steps with 25.0 mL of the 1.0 M HCl solution and 25.0 mL of the 1.0 M NaOH solution. I noted the maximum temperature and tidied up the workbench.
Results
In the investigation, a known weight of reactants is accompanied by many solutions. Then, the thermal change in the solutions column is recorded by the virtual thermometer. Temperature change, ΔT = T final – T initial, is measured in kilojoules (kJ) as the unit of measuring energy change, and temperatures (T) were measured in degrees (°C). The data table with the appropriate values for each entry is shown below.
Sample Calculations
Trial 1
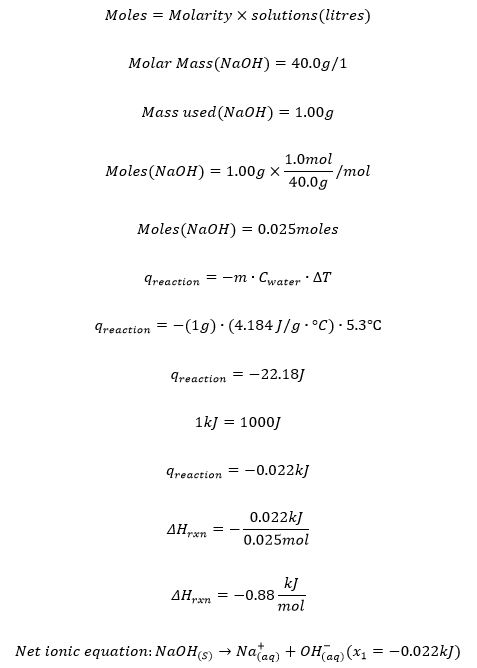
Trial 2
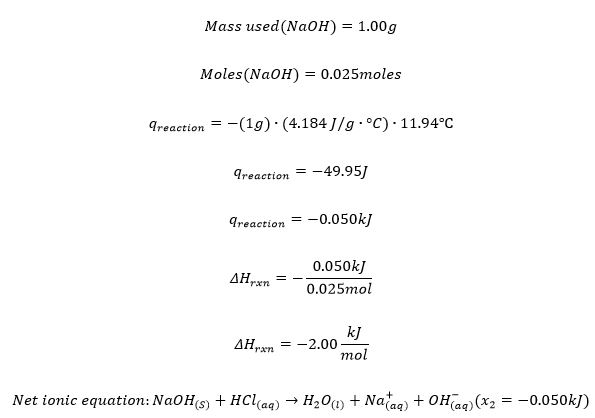
Trial 3

Discussion
The investigation results demonstrated that the percentage difference between the reactions was 0%. This indicates that the values obtained comply with the accepted values, and the trials had minor occurrences of any uncertainties. The results of these investigations also demonstrate that all the reactions are exothermic.
Since all reactions generate heat, they cannot establish the validity of the Hess Law. Hess’s Law requires calculated enthalpies to be constant(Pecchi et al., 2020). Therefore, from the calculated values of x4 and x5, it is validated that the chemical reactions obey Hess’s Law since the enthalpies are constant.
Summary
Understanding Hess’s Law of thermochemistry is crucial as it enables the formation of new chemical equations whose variations in enthalpy can be calculated instead of being directly measured. This is useful in this investigation because units such as joules and kilojoules cannot be measured directly. Understanding exothermic reactions and demonstrating how heat is distributed during them reveals how they function.
Reference
Pecchi, M., Patuzzi, F., Basso, D., & Baratieri, M. (2020). Enthalpy change during hydrothermal carbonization of biomass: a critical review. Journal of Thermal Analysis and Calorimetry, 141(4), 1251-1262. Web.