A detailed study of the regularities of chemical molecule interactions at both the molecular and electronic levels allows summarizing the available knowledge and identify key trends qualitatively. Among others, it is of research value not only to generalize but also to identify the potential of reaction pathways and bias effects initiated by catalysts or other added substances. Finally, an excellent demonstration of personal knowledge and competence are assignments in which the student is asked to propose organic synthesis schemes using only the substrate as the source. Summarizing all of the above, it is essential to note that solving such assignments is a sound academic strategy for learning chemical sciences. At the same time, aromatic substances that have a benzene ring in the molecular structure have the highest stability due to the balanced electron density throughout the sp2-hybridized ring. Consequently, it is the study of aromatic substances that is of great importance to all organic chemistry. Therefore, this essay consistently discusses the answers to the four proposed assignments on the topic of organic synthesis. This paper is valuable and relevant material not only for the author but also for all students interested in finding answers to organic synthesis questions.
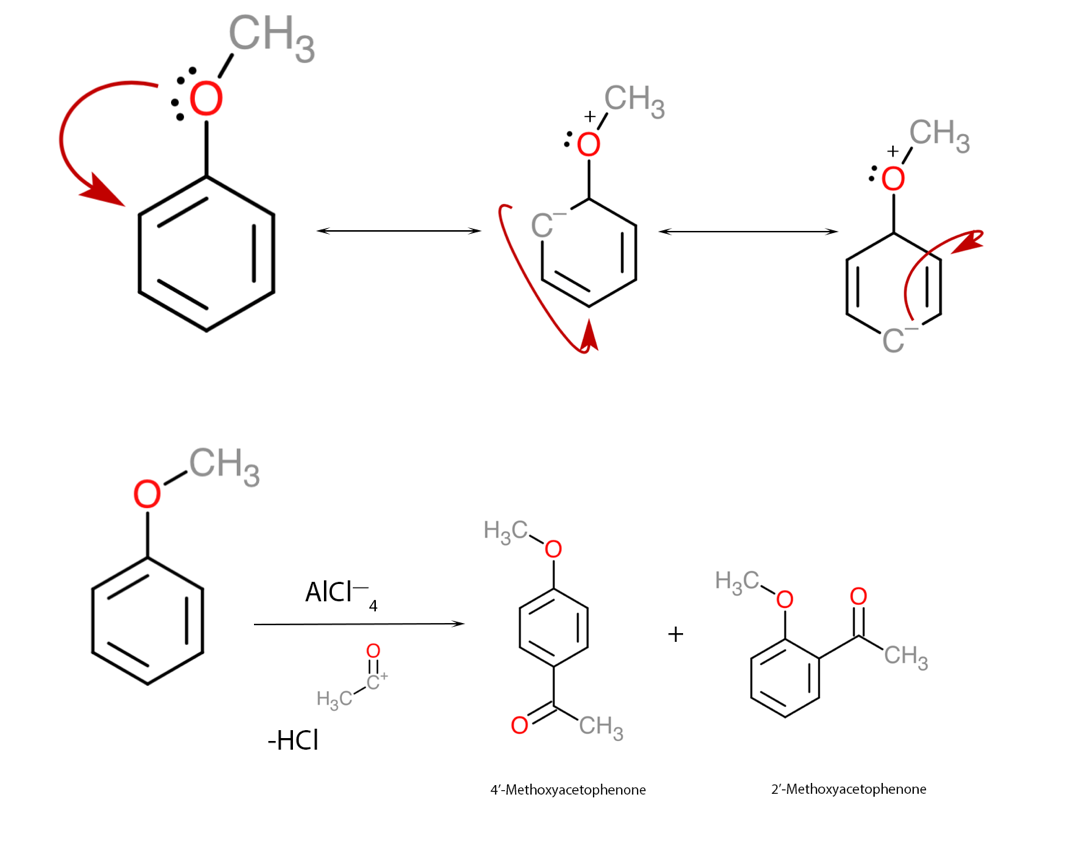
First of all, it should be said that aluminum chloride, also known as Lewis acid, is often the substance that initiates and catalyzes the reactions of organic addition of electrophilic substituents to the π-electron cloud. The addition of foreign atoms or atomic groups causes the electron density of the cloud to shift and move throughout the ring until maximum stability is achieved. The methoxy group in this assignment is a substituent that transfers electrons into the ring and thus increases the overall electron density of the aromatic ring. In addition, the final position of the substituents must be found, including the steric effect that prevents the effective course of chemical reactions. Concerning anisole, Lewis acid initiates addition at the ortho- and para-positions, and therefore it should be expected that the methoxy group nucleophile to join at both positions, forming two reaction products: 2-methoxyacetophenone and 4-methoxyacetophenone, as shown in Figure 1. In this case, 4-methoxyacetophenone has a higher yield due to the optimal location of the substituent minimizing the steric effect.
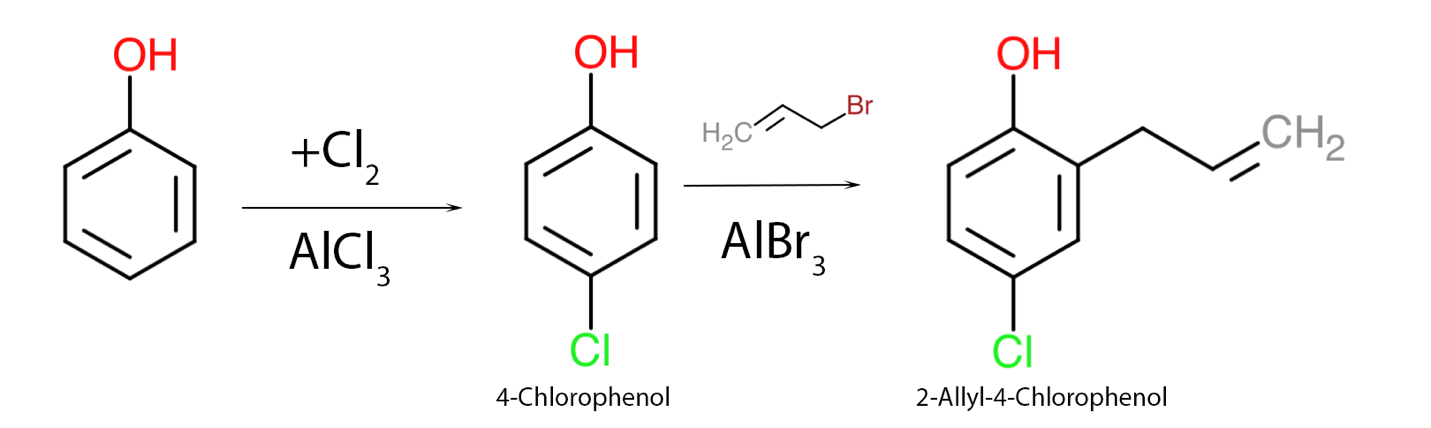
The second assignment, which assesses the student’s academic competence, examines possible ways of synthesizing one organic substance from another. More specifically, for this essay, phenol as a source and 2-allyl-4-chlorophenol as the final product were chosen. It is already clear from the product name that nucleophilic substituents at the ortho- and para-positions must be added to the benzene ring. For this purpose, a series of Friedel-Crafts organic mechanisms with Lewis acid as a catalyst should be used (Ashenhurst, 2021). It should be noted, nevertheless, that the halogen in the added salt should correspond to that in the nucleophile, as shown in Figure 2. Again, positions 2 and 4 were driven primarily by stabilizing the electron density of the π-cloud and minimizing the steric effect. Thus, two nucleophilic substitution reactions are sufficient to obtain 2-allyl-4-chlorophenol from ordinary phenol.
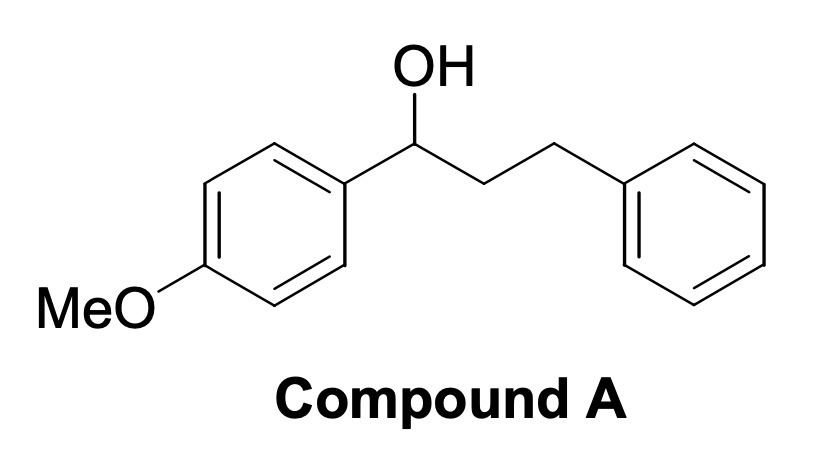
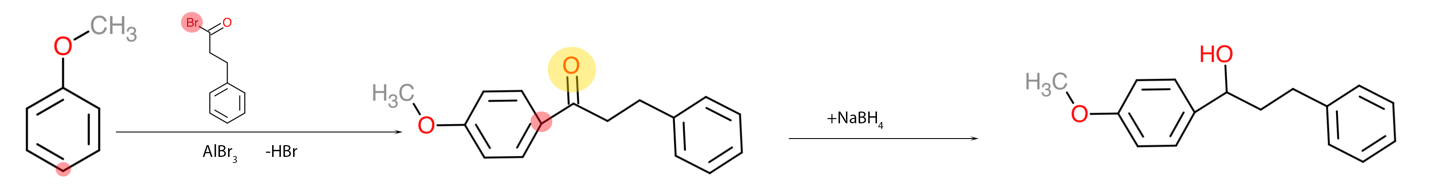
It is also imperative to be able to obtain complex organic substances from simpler ones since this task has practical applications. For example, if a particular substance is not enough in the laboratory, it can be obtained by organic synthesis reactions and then used as intended. The third task belongs to this category because it asks about the potential pathway for synthesizing complex compound A — Figure 3 — from anisole, shown in Figure 1. It can be seen that the number of aromatic rings has doubled, with only the left corresponding to the original anisole. Hence, it is appropriate to use the Friedel-Crafts reaction to attach a massive nucleophile to the para-position with the corresponding Lewis acid. Once the intermediate molecule is obtained, the double oxygen bond must be reduced so that a hydroxyl group is obtained from the carbonyl group (Kennepohl, 2019). A semi-anionic hydride acting as a nucleophile transferring hydrogen to the carbonyl carbon can be used for this purpose. The final processes are shown in Figure 4.
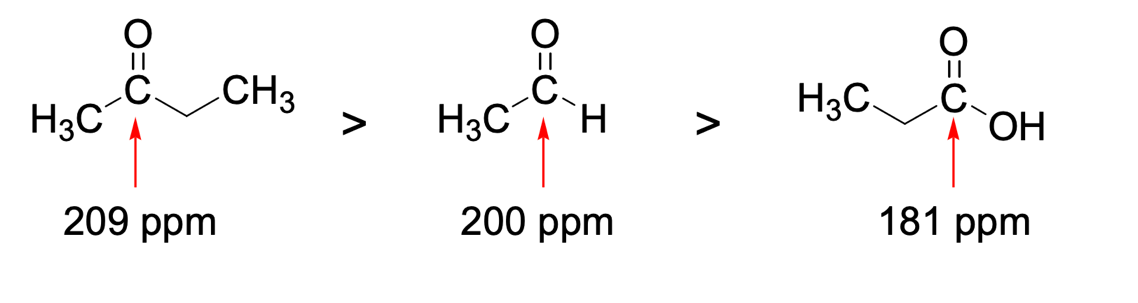
Finally, in the organic chemistry section, the use of instrumental methods of analysis that allow qualitative identification of compounds is of great importance. NMR forms the basis of such methods since it gives a highly accurate and sensitive understanding of the nature of the molecule. NMR performed on 13C isotope nuclei generally allows suitable identification of the parts of the substance, and especially the aromatic components. As shown in Figure 5, the specific signal value for the same carbon atom surrounded by different groupings also varies. More specifically, the quantitative signal measure — ppm — decreases in the following sequence: ketones, aldehydes, acids. The reason for this shift, caused by the carbon environment, may be the amount of free hydrogen in the neighborhood of the selected carbon atom. More specifically, in ketones, carbon has five alpha hydrocarbons — three on the left and two on the right — in aldehyde only three — all on the left — and finally, in acid, carbon has only two alpha hydrocarbons — both on the left. Thus, the lower the number of hydrocarbons a selected carbon has, the lower the ppm number for it. This is not surprising since the chemical shift value is based on the number of equivalent hydrocarbons (Clark, 2020). The more hydrogen surrounding the atom, the more to the right it shifts on the horizontal axis. The figure below confirms these beliefs. Thus, using personal chemical knowledge and competence can solve complex problems.
References
Ashenhurst, J. (2021). EAS reactions (3) – Friedel-Crafts acylation and Friedel-Crafts alkylation. Master Organic Chemistry. Web.
Clark, J. (2020). Interpreting C-13 NMR spectra. LibreTexts. Web.
Kennepohl, D. (2019). Reduction. LibreTexts. Web.