Introduction
The absorbance of a substance can be measured by determinig the absorption spectra of the substance. A spectrometer can be used to measure the energy transition from the ground state of a substance to an excited state of the substance which is usually above 300KJmol-1(Bhowmik et al., 2018).The absorbance spectra of the two dyes which were 1,1-diethyl-2,2-carbocyanine iodide and 1,4-diphenyl-1,3-carbobutadiene were measured and recorded. The following experiment will focus on performing the spectroscopy of1,1-diethyl-2,2-carbocyanine iodide and1,4-diphenyl-1,3-carbobutadiene. The report will also analyze and discuss the results from the experiemnt.
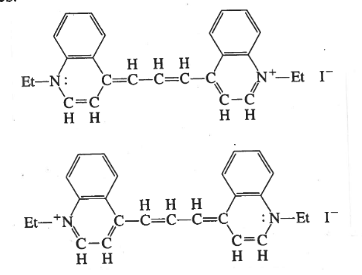
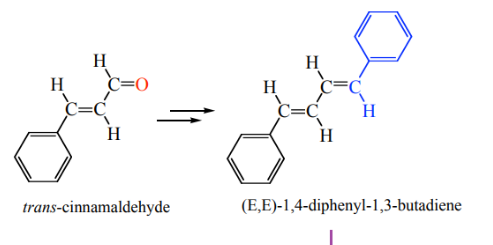
Procedure
- 10ml of methyl alcohol was first prepared as a solvent at a molarity of 10-3M to deteremine the absorbance band of the standard soluton.
- 10ml solution of the dyes were also prepared at a molarity of 10-3M and their spectra were measured.
- The results of absorbance of 1,1-diethyl-2,2-carbocyanine iodide and 1,4-diphenyl-1,3-carbobutadiene were later recorded
Results
The results of the absorbance of 1,1-diethyl-2,2-carbocyanine iodide and 1,4-diphenyl-1,3-carbobutadiene were taken and recorded in the table below.
The result of the following data shows that 1,1-diethyl-2,2-carbocyanine iodide had more absorbance spectra than 1,4-diphenyl-1,3-carbobutadiene.The absorbance of methyl alcohol is 184nm, hence the absorbance of the two dyes was relatively higher than that of the standard solution used in the spectrometry. The dyes in the following experiment showed a high absorbance, which means they can be used to form good coloring matter.
Analysis
The quality of a dye can be measured by determining its absorbance. The absorbance spectra of the following two dyes showed values of 614nm and 848nm for 1,1-diethyl-2,2-carbocyanine iodide and1,4-diphenyl-1,3-carbobutadiene respectively. The two dyes can therefore be used to develop a quality coloring matter. 1,4-diphenyl-1,3-carbobutadiene however, shows a higher absorbance hence is a prefferable dye than 1,1-diethyl-2,2-carbocyanine iodide. 1,4-diphenyl-1,3-carbobutadiene can easily penetrate on a surface more than 1,1-diethyl-2,2-carbocyanine iodide. The absorbance of methyl alcohol was relatively low, hence cannot be used as a dye.
Discussion
Spectroscopy is a method used to measure the absorbance of substances which also deteremines the spectra of the substances. The absorbance measures the ability of a substance to absorb and transmit energy when inserted in a spectrometer (Bhowmik et al., 2018). The transmission of energy from a substance or molecule begins from the ground state and is excited until it reaches the maximum energy level.
The differences in the energy used during the transmission of energy from the ground state to the maximum absorbance can be used to determine the spectra of the substance (Bhowmik et al., 2018). The following experiment was conducted to determine the absorbance of 1-diethyl-2,2-carbocyanine iodide and 1,4-diphenyl-1,3-carbobutadiene. The results show that 1,4-diphenyl-1,3-carbobutadiene has more absorbance than 1,1-diethyl-2,2-carbocyanine iodide, which means the substance has more energy to penetrate of surfaces. The dyes in the following experiment are suitable for use since they have an absorbance of more than 300nm.
Conclusion
Spectrometry can be used to measure the absorbance of substances such as dyes. The following experiment was used to measure the absorbance of 1,1-diethyl-2,2-carbocyanine iodide and 1,4-diphenyl-1,3-carbobutadiene. The results of the absorbance were tabulated for comparison. It was found that 1,4-diphenyl-1,3-carbobutadiene had more absorbance than 1,1-diethyl-2,2-carbocyanine iodide. The following experiment found that both dyes had an absorbance of more than 330nm which means dyes had more adequate absorbption energy. The following study was was conducted severally to find accurate measurements.The dyes used in the following experiment were found to posses good quality because of their high absorbance values of more than 330nm.
Reference
Bhowmik, K. L., Deb, K., Bera, A., Debnath, A., &Saha, B. (2018). Interaction of anionic dyes with polyaniline implanted cellulose: Oganic π-conjugated macromolecules in environmental applications. Journal of Molecular Liquids, 261, 189-198. Web.