Definition
Bordetella Pertussis is classified as a Gram-negative anaerobic bacterium. It is defined as an encapsulated coccobacillus and belongs to the Bordetella genus (Finger and Koenig par. 1-2).
Signs and Symptoms
The bacterium causes the development of Pertussis in people. The disease manifests itself in mild cough, frequent sneezing, rhinorrhea, and mild fever (Centers for Disease Control and Prevention “Signs and Symptoms” par. 2).
Classifications
As a rule, B pertussis and B parapertussis are typically identified when considering the typology of the bacteria.
Diagnosis
The process of disease contraction occurs by droplets. In other words, the disease is classified as respiratory. The bacteria can be cultured in the Stainer-Scholte broth. The detection of the disease, in its turn, can be carried out by applying the PCR tool. It should be borne in mind that the first (i.e., the catarhal) stage of the disease progress is the most infectious one. Therefore, the patients should follow the security measures that will provide the rest of the members of the community with safety and prevent the further contraction of B. pertussis. The infectiousness of the disease is retained for at least five weeks, after which the patient experiences a gradual improvement.
Treatment
Though rather common, the erothromycin treatment does not lead to the elimination of the disease symptoms and the removal of the bacteria from the patient’s organism. Instead, the identified treatment tool serves as the means of reducing the infection period from the five weeks mentioned above to 5-10 days (Tiwan par. 4). However, the Centers for Disease Control and Prevention (2016) advise to use the medicine identified above as one of the essential means of addressing the development of B. pertussis in children; specifically, the application of the medicine such as “erythromycin, clarithromycin, and azithromycin” (Centers for Disease Control and Prevention “Pertussis. Whooping Cough” par. 3) is strongly suggested.
Complications
Seeing that B. pertussis is a disease that affects primarily the respiratory system, it is reasonable to suggest that the development of severe respiratory issues should be listed among the key complications to be expected. Indeed, the existing studies point to the fact that bronchopneumonia and acute encephalopathy are caused by the subject matter unless the corresponding medicine is administered to the patient in a timely manner.
The issues mentioned above should be taken with due seriousness as they may result in the patient developing serious health issues and even dying. According to a recent research, the identified complications may cause a severe and lifelong brain damage unless addressed in an appropriate manner. In addition, acute encephalopathy is likely to cause convulsions (Finger and Koenig par. 2-3).
Preventions
The tools for preventing B. pertussis are rather numerous, yet vaccination is doubtlessly the most efficient one. However, it would be wrong to assume that B. pertussis requires a separate vaccine; instead, the injection addresses several infections, such as diphtheria and tetanus. As a rule, the injection is provided to patients at a comparatively early age. However, unlike other types of vaccines, the one against B. pertussis is carried out in several stages. The patients are vaccinated at the age of 2, 4, 6, 12-15 months, and 4-6 years (Centers for Disease Control and Prevention “Diphtheria, Tetanus, and Pertussis Vaccine Safety” par. 104).
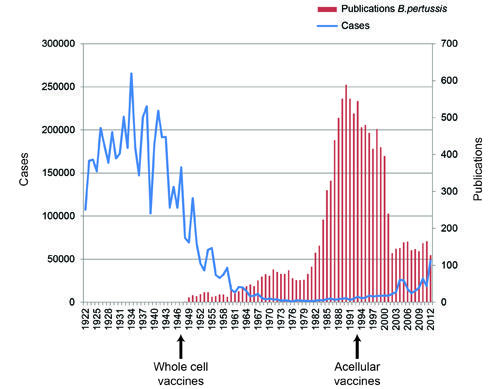
Statistics
The instances of B. pertussis contractions are rather few. Nevertheless, the disease still remains a threat, as Fig. 1 shows. Therefore, it is imperative that vaccinations against B. pertussis and the related bacteria should be viewed as compulsory.
Works Cited
Bordetella Pertussis Vaccine Development 2016. Web.
Centers for Disease Control and Prevention 2016. Diphtheria, Tetanus, and Pertussis Vaccine Safety. Web.
— 2016. Signs and Symptoms. Web.
— 2016. Pertussis (Whooping Cough). Web.
Fingerm, Horst, and Carl Heinz von Koenig 2012. Chapter 31. Bordetella. Web.
Tiwan, Tejpratap 2015. Recommended Antimicrobial Agents for the Treatment and Postexposure Prophylaxis of Pertussis. Web.