Abstract
The study of pH has an increased value for clinical studies, mainly due to the buffering properties of some substances. The present experiment tested buffering for three samples: water, Alka-Seltzer, and milk. The results showed that Alka-Seltzer is characterized by increased buffering because it is most resistant to changes in external acidity. This indicates the enhanced clinical efficacy of Alka-Seltzer in resisting external pH to perform targeted therapeutic actions.
Introduction
The study of the chemical properties of a substance is characterized by increased practical value, especially for clinical and pharmaceutical purposes where the matching of the administered substance with human tissues and organs is of critical importance. For the present experiment, the focus was set on pH values, which correspond to the hydrogen ion content of a solution – the more protons present, the lower the pH of the medium, and the more acidic the solution is, and vice versa (Swietach, 2019). pH compatibility has particular importance in the clinical industry, as the medications used must perform the intended action but not destroy the organ microflora, leading to an acid-base imbalance (Kuo et al., 2020).
In the present work, the pH of two different solutions, ordinary cow’s milk, and Alka-Seltzer tablet, is investigated, aiming to analyze the dynamics of the acidity index change when hydrochloric acid is added. A substance is characterized by good clinical properties if it maintains its pH relatively stable in the presence of an external stimulus. The present experiment was thus based on the hypothesis that the change in pH for Alka-Seltzer as HCl and NaOH were added would not be as dynamic as for milk or water (control) not used for clinical purposes. The study predicted that if Alka-Seltzer had the greatest buffering properties, then the pH changes of this solution would not be as dynamic.
Materials and Methods
The study was based on an experiment in which the type of solution under study (Alka-Seltzer, milk, water) was the independent variable. The dependent variable was pH, which was evaluated using a pH meter at regular intervals when three successive drops of HCl and NaOH were added. The control and standardized variables of the experiment were temperature and atmospheric conditions, which were not changed, and the calibrated pH meter. To prepare Alka-Seltzer, one tablet of the drug was dissolved in 118 mL water in a 200-mL beaker. Next, 25 mL of milk was transferred to a 50-mL beaker. First 1M HCl and then 1M NaOH was added to each solution in three drop increments, and the results of pH measurement were recorded for each step in a table.
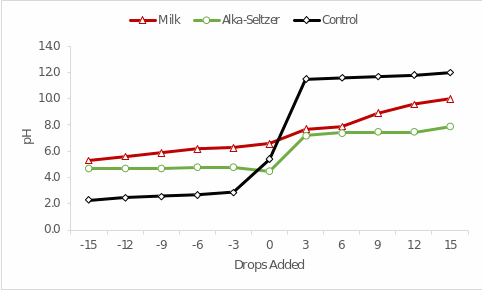
Results
The measurement results are shown in Table 1 below: without intervention, the pH of the milk was determined to be 6.6, the Alka-Seltzer pH was 4.5, and the control was 5.4. The pH data and the number of drops added were plotted on the graph shown in Figure 1. The visualization shows that as the pH of the added reactants decreased (from acid to alkali), the pH of each of the samples studied increased, but at different rates. The absolute magnitude of the pH change was minimal for Alka-Seltzer (∆1 = 3.4) and increased for milk (∆2 = 4.7) and control (∆3 = 9.7).
Table 1. Results of pH measurements.
Discussion
The experiment hypothesized that Alka-Seltzer has enhanced buffering properties compared to milk and water (control). The change in pH for Alka-Seltzer was minimal, meaning that the substance maintained its relative stability when the medium’s acidity changed by 32.1% compared to milk and 69.44% better than water. This implies that the hypothesis of buffering properties of Alka-Seltzer was confirmed, and of the samples studied, Alka-Seltzer tablets did have an increased resistance to external stimuli.
The study could be inaccurate due to some errors, including inaccurate pH meter measurements, impurity structures in the samples’ composition, and errors in recording the data. Working to eliminate these may be part of a future experimental extension. In addition, it would be appropriate to add other medications to the study, including defoamers and heartburn-relieving agents, to evaluate their buffering properties against marketing claims of efficacy.
References
Kuo, S. H., Shen, C. J., Shen, C. F., & Cheng, C. M. (2020). Role of pH value in clinically relevant diagnosis. Diagnostics, 10(2), 1-7. Web.
Swietach, P. (2019). What is pH regulation, and why do cancer cells need it? Cancer and Metastasis Reviews, 38, 5-15. Web.