Materials and Methods
A stock solution of starch was prepared by mixing 1ml of starch with 2.5ml of hydrogen peroxide (H2O2) to form 3.5ml of the stock solution, which is enough for the experiment. Next, six test tubes were arranged in their rack and labeled one to six. To prepare the iodine solution, 250µl of potassium iodide (KI) was added into each test tube. Increasing amounts of vitamin C (ascorbic acid) were added into each test tube from the second test tube to the sixth test tube as 25µl, 50µl, 75µl, 100µl, and 200µl respectively. The first test tube was left blank to act as a control for the experiment. All the six test tubes were then topped up with the respective amounts of water for the total solution to be 450µl to create a concentration gradient of ascorbic acid. The table 1 below indicates how the reagents were placed into each of the six test tubes to form an iodine solution.
Table 1
In the reaction of starch with iodine solution, 350µl of stock solution was added to each of the test tubes that have an iodine solution at a time. Observations were made, and the stopwatch was used to measure the time taken in seconds for the color of the reactants to change to blue-black. The table 1 below illustrates the amount of iodine solution and stock solution, adding up to 800µl when mixed as reactants.
Table 2
To enhance the validity and reliability of the findings, the whole experiment was repeated three times, and the average period in seconds was determined and provided for further analysis. The average time is taken for the color to change to happen calculated from the three readings and tabulated. The findings were used to construct a graph showing the time taken for the color to change versus the amount of ascorbic acid. The graph was then used to determine the amount of ascorbic acid necessary to cause colour change in 40 seconds.
Data
The data obtained from the three experiments was recorded in the following table. The table indicates the time that colour development took in each of the test tubes in all the three experiments increases according to the amount of ascorbic acid.
Table 3
In the construction of the graph, an average period was determined in relation to the amount of ascorbic acid in each of the test tubes. Table 4 shows the relationships between the average time taken for the colour change to occur and the amount of ascorbic acid used.
Table 4
The results in table 4 were used to construct a graph, which depicts the relationship between time taken for colour change to occur and the amount of ascorbic acid that delayed the occurrence of the colour change.
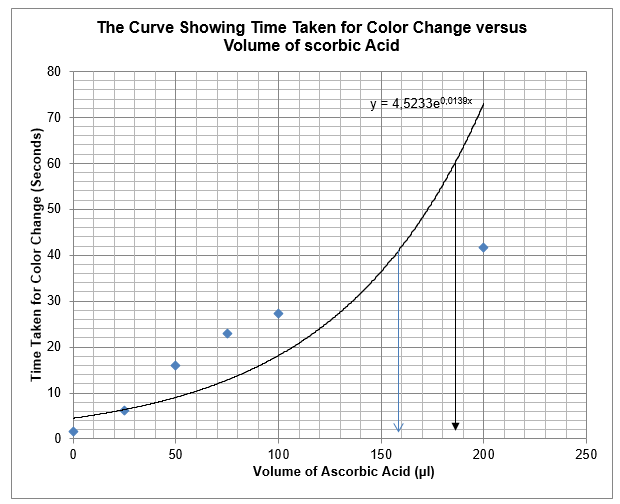
From the graph, the amount of ascorbic acid that can cause colour changes to occur after 40 seconds is about 158µl. Moreover, if the colour changes should occur after 60 seconds, about 185µl of ascorbic acid are necessary.
The use of the exponential equation provides an accurate amount of ascorbic acid needed to cause colour changes. The exponential curve has the equation y = 4.523e0.013x.
Results and Discussion
The results indicate that the amount of ascorbic acid in the iodine solution determines the occurrence of colour changes when iodine and starch react. Usually, iodine reacts with starch to form a complex that gives blue-black colour. According to Pommerville (2010), the reaction between starch and iodine in presence of hydrogen peroxide result in the formation of a blue-black complex. For the iodine to react with starch, hydrogen peroxide must oxidize it into triiodide (Aklima, Mojumder, & Sikdar 2014). Triiodide reacts readily with starch molecules yielding a blue-black colour, which has become a standard test for detecting the presence of starch in various substances. Thus, in the preparation of stock solution, hydrogen peroxide is added to the starch so that when it encounters iodine ions in solution, oxidation starts.
The following chemical equations explain what occurs when the iodine ion solution encounter starch in the presence of hydrogen peroxide.
The first equation
Hydrogen peroxide + Iodine ions in presence acid → Triiodide ions + Water
The second equation
Starch + Triiodide ions → yield blue-black complex of starch and triiodide
The equation indicates that the presence of hydrogen peroxide is necessary for iodine ions to convert into triiodide, a product that reacts with starch. BeMller and Whistler (2009) argue that the conversion of iodine ions into triiodide is rate-limiting step because it determines the reaction of starch and iodine. The reaction of starch and iodine cannot take place if triiodide ions are not present. This means that hydrogen peroxide should be in excess for the iodine to react with starch optimally.
In the first test tube, it is evident that the starch and iodine reacted immediately and formed blue-black complex because ascorbic acid was not present. It took an average of 1.7 seconds for the colour changes to happen in the first test tube. However, the presence of ascorbic acid in subsequent test tubes reduced the rate of colour formation. According to Vieira et al. (2010), ascorbic is a strong reducing agent that reduces triiodide ions into iodine ions making them unavailable for the reaction with starch.
Triiodide ions + Ascorbic acids → Iodine ions + dehydroascorbic acid
For the colour formation to occur, triiodide ions must oxidize ascorbic acid into dehydroascorbic acid, which is a product that is unable to reduce triiodide. Oxidation of ascorbic acid reduces its reducing power and thus prevents it from reducing triiodide ions into iodine ions (Babatunde & Nwakama 2013). In this view, the reactions between starch and iodine delayed in the subsequent test tubes because ascorbic acid was using available triiodide ions. After the ascorbic acid was oxidized completely, colour formation started to happen because triiodide became available for the reaction to proceed. This means that the amount of ascorbic acid present in the iodine solution determines the duration of colour formation.
The time taken for the colour changes to occur increased as the concentration of ascorbic acid increases. With the amount of ascorbic acid as 25µl, 50µl, 75µl, 100µl, and 200 µl were 6.3, 16, 23, 27.3, and 41.7 seconds respectively. Apparently, the increase in time as per the increase in the amount of ascorbic seems to have linear relationships. However, the drawing of the graph depicts the time taken for the formation of colour and the amount of ascorbic acid has exponential relationship. This implies that the time taken for the colour formation in iodine solution that has large amount of ascorbic acid could be infinite. Zhang (2012) asserts that excess ascorbic acid reduces triiodide ions and prevents the formation of blue-black colour. Hence, ascorbic acid should not be in excess to allow the formation of triiodide ions and their reaction with starch.
From the graph, the amount of ascorbic acid necessary to cause colour changes after 40 seconds is about 158µl. To carry out this test, 158µl of ascorbic acid would be added to 42µl of water, and then added 250µl of potassium iodide to form iodine solution. Reaction of 450µl of iodine solution and 350µl of starch solution would yield colour in approximately after 40 seconds. The predicted and verified values were not significantly different as the values were 158µl and 200µl respectively. The discrepancy occurred due to errors in timing and measurement of reagents. Since hydrogen peroxide and iodine solution are limiting reagents, I would put them in excess in the future because they have a great potential to confound the experimental results.
Extra Findings
From the experiment, one can determine the amount of ascorbic acid necessary to cause changes in colour. It requires 185µl of ascorbic acid for the colour changes to happen after 60 seconds. This implies that 185µl of ascorbic acid will be added to 15µl of water, then added 250µl of potassium iodide.
To determine the amount of ascorbic accurately without using the graph, an exponential equation is applicable. The exponential equation of the curve is y = 4.523e0.013x. Where y is the time that it would colour change to occur and x is the amount of ascorbic acid.
References
Aklima, J, Mojumder, S, & Sikdar, D 2014, ‘Total phenolic content, reducing power, anti-oxidative, and anti-amylase activities of five Bangladeshi fruits’, International Food Research Journal, vol. 21, no. 1, pp. 119-124. Web.
Babatunde, A, & Nwakama, N 2013, ‘Kinetic approach to the mechanism of oxidation of L-ascorbic acid by periodate ion in acidic medium’, Journal of Oxidation of Biology and Life Sciences, vol. 4, no. 2, pp. 32-42. Web.
BeMller, J & Whistler, R 2009, Starch: Chemistry and Technology, Academic Press, New York. Web.
Pommerville, J 2010, Alcamo’s Laboratory Fundamentals of Microbiology, Jones & Bartlett Publishers, New York. Web.
Vieira, C, Milani, V, Lisboa, V, Menezes, C, Jorge, L, & Giglio, J 2010, ‘Further insights toward vitamin C determination and stability: Proposal of a new quantification method’, Bioscience Journal of Uberlandia, vol. 26, no. 2, pp. 296-304. Web.
Zhang, Y 2012, Ascorbic Acid in Plants: Biosynthesis, Regulation, and Enhancement. Springer, New York. Web.