Introduction
The use of analytical chemistry tools helps to improve the accuracy of results and solve laboratory problems of qualitative identification, determination of concentrations, and composition of unknown components. In the present experiment, the chemical analysis tool is chromatography, which is a method of separating and analyzing a mixture of substances based on differences in molecular weights and dissolution abilities. Column chromatography is based on the different affinity of the components of a mixture to the substance the column is filled with; some of the components are trapped in the sorbent, while others pass freely through it (Hinterberger et al., 2019).
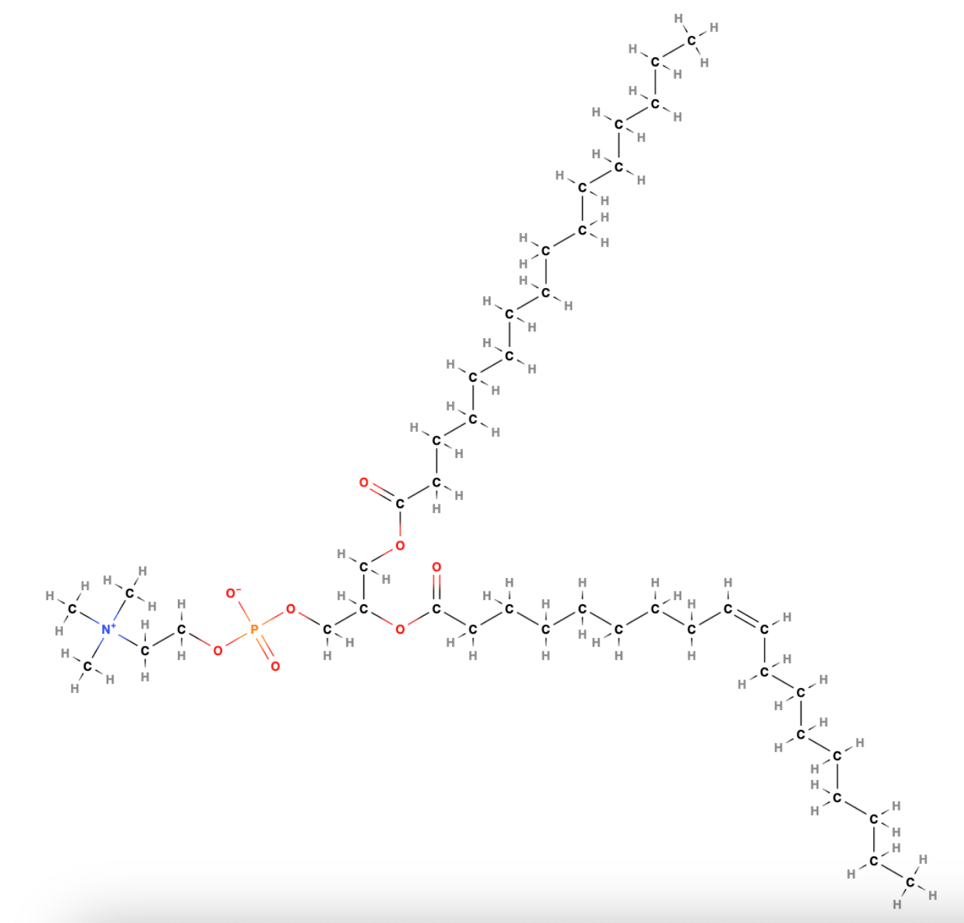
Thin-layer chromatography is an alternative type of chemical analysis in which the components of a mixture are applied to a thin layer of sorbent and placed in an eluent, where they are separated at different rates by gravity (Poole, 2023). In addition, there is gas chromatography, in which components of a mixture are separated in the gas phase based on their interaction with the stationary phase, molecular weight, and dissolution ability (Zoccali et al., 2019). In the present laboratory work, three types of chromatography are used to obtain and analyze Phosphatidylcholine phospholipid (Figure 1) derived from egg yolk.
Experiment
Reagents and Materials
- Egg yolk.
- Distilled water.
- Acetone.
- Potassium hydroxide (KOH).
- Methanol.
- Hydrochloric acid (HCl).
- Chloromethane (liquid).
- Hexane (liquid).
- Aluminum oxide (solid).
List of Equipment
- Blender.
- Vacuum filter, clamp, and tripod.
- Buechner funnel.
- Paper Filter.
- Erlenmeyer Flask 100ml.
- Ice bath device.
- Magnetic stirrer.
- Tared flask.
- Glass flask (2X40cm), glass wool stopper.
- 100 mL glass flasks (4 pcs).
- Calibrated flasks.
- TLC plate with a thin layer of silica gel.
- Oven.
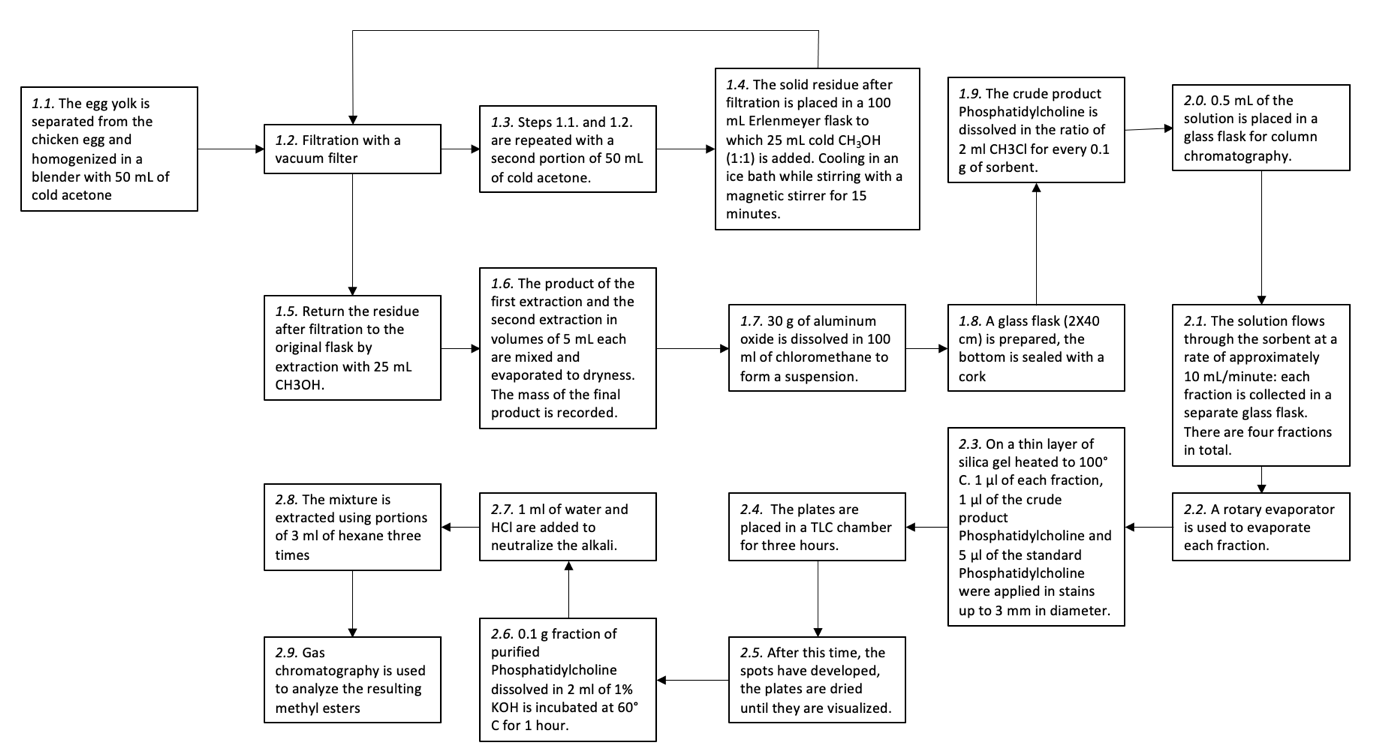
The lists above contain the materials and reagents used for all experiment stages, as well as the laboratory equipment used. The experiment consisted of three parts: the first extracted the crude Phosphatidylcholine from the egg yolk, the second performed column and thin-layer chromatography, and the third used gas chromatography to identify the purity of the resulting product. The figure below shows the procedural protocol of all phases of the experiment.
Data and Calculation
Table 1 contains the gas chromatography results, calculating the fraction of each fatty acid ester detected. Three residues, namely methyl palmitate, methyl stearate, and methyl linoleate, were detected in the purified sample. As shown in the table, the highest composition was characteristic of Methyl stearate.
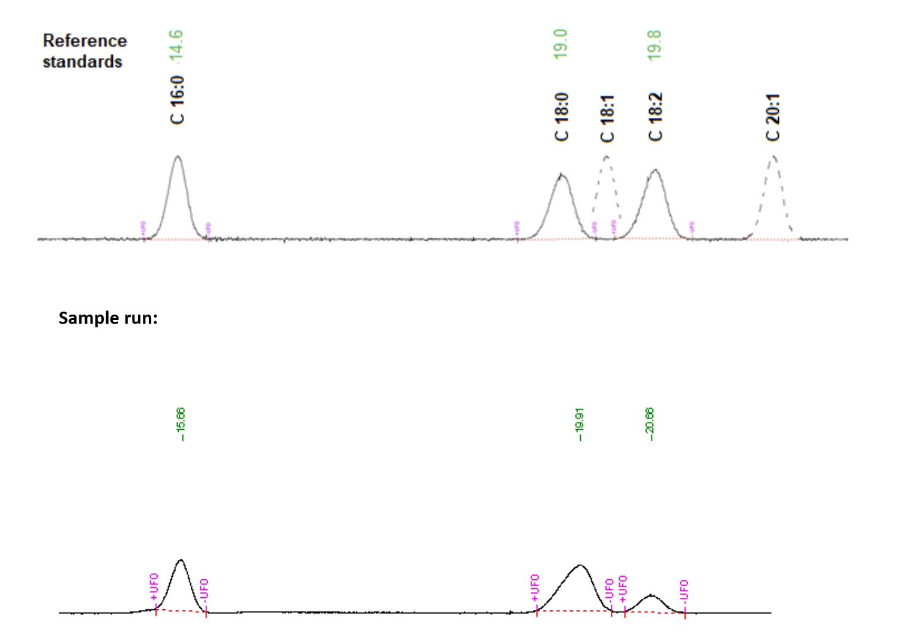
Figure 3 shows the gas chromatography results visualized as spectra. As the data indicate, the three expected peaks (C 16:0, C 18:0, and C 18:2) were detected, but their intensities, that is, the area under the curve, were higher than in the reference standard.
Results and Discussion
The purpose of this work was to isolate and analyze the Phosphatidylcholine composite using chromatography procedures. The results showed that the purified Phosphatidylcholine sample contained the expected fatty acid residues with the highest concentration of Methyl stearate. The observed shift in peak intensities from the gas chromatography could indicate an increased concentration of each residue compared to the reference standard, but could also result from a systematic instrumental error.
Based on the results, we can conclude that in different quantitative ratios, all three fatty acid residues (palmitate, stearate, and linoleate) were included in Phosphatidylcholine. This correlates with the expected results because Phosphatidylcholine is a phospholipid that includes different types of fatty acids at the sn-1 and sn-2 positions of the glycerol backbone (Bradley et al., 2022). Since each Phosphatidylcholine molecule consists of two such acid residues, it cannot be excluded that a combination of different types of such molecules was observed in the egg yolk.
Conclusion
To summarize, the experiment can be considered a success, as it achieved the result and satisfied the research goal set at the beginning. Nevertheless, due to time constraints, the third part of the experiment, related to gas chromatography, was not performed, so old data were used. Thus, part of future research is to extend the timeframe of the experiment with in-house gas chromatography and use repeated tests to reduce the likelihood of error and instrumental errors.
References
Bradley, R. M., Hashemi, A., Aristizabal-Henao, J. J., Stark, K. D., & Duncan, R. E. (2022). PLAAT1 exhibits phosphatidylcholine: Monolysocardiolipin transacylase Activity. International Journal of Molecular Sciences, 23(12), 1-12. Web.
Hinterberger, V., Damm, C., Haines, P., Guldi, D. M., & Peukert, W. (2019). Purification and structural elucidation of carbon dots by column chromatography. Nanoscale, 11(17), 8464-8474. Web.
Poole, C. (2023). Instrumental thin-layer chromatography. Elsevier.
Zoccali, M., Tranchida, P. Q., & Mondello, L. (2019). Fast gas chromatography-mass spectrometry: A review of the last decade. TrAC Trends in Analytical Chemistry, 118, 444-452. Web.