Case Study
Melissa Moser, a female aged 22 years indicated symptoms of unconsciousness and shortness of breath when she was first admitted to the community hospital. The patient data as recorded on admission showed that she was 70kgs heavy, 66 inches tall and her BSA was 1.81m2; two weeks ago Melissa was diagnosed with pneumonia-type symptoms, treated, and discharged. Immediately after arriving at the hospital, the patient suffered a cardiopulmonary arrest that required doctors to apply cardiopulmonary resuscitation (CPR), mechanical ventilation and inotropic support in order to resuscitate her.
Soon she was stabilized and a palpable purse established at which point she was subjected to a chest CT scan and an echocardiogram (echo) scan. The result of the echo scan indicated the presence of an element that was mobile within the heart which had caused dysfunction in the right ventricular and under-filling of the left ventricular. The CT scan results also showed excessive pulmonary embolism at the right pulmonary artery and within the distal of the left main pulmonary artery, similar to symptoms of Saddle Embolism (Chappel 2006)
Pathology
Pulmonary Embolism (PE) is a disease condition that is caused by blockage of any of the main arteries to the lungs by a mobile element referred as embolism in medical terms which could either be cholesterol particles, blood clots, bone fractures, fat droplets, air, bacteria pus, hair, fetal cells or any other foreign objects. It is this blockage that abstracts blood flow to the right ventricle of the heart which is responsible for the various symptoms that characterize the disease condition.
As such, PE symptoms usually include difficulty in breathing, rapid heart rate, chest pains, low-oxygen saturation in blood, perspiration, reduced blood pressure and sometimes fever (Righini 2010).
There are two major risk factors that are responsible for the majority of PE conditions; pelvic vein thromboses and deep venous thrombosis (DVTs) (Righini 2010). This means that all the other associated risk factors of the two conditions are also indirect risk factors of PE which are collectively referred as venous thromboembolism (VTE) (Righini 2010). This is because all forms of venous clot that occur in blood poses a threat of PE in situations when they get dislodged from the blood veins and get transported within the lungs which is the case for a significant proportion of cases.
The predisposition of a person to suffer from any form of thrombosis is again attributed to another group of factors that are referred as Virchow’s Triad; these are physical characteristics in blood arteries and its properties that significantly heighten the likelihood of thrombosis to occur in an individual (Righini 2010). They include such factors as a change in blood pressure and flow, the nature of blood vessels and unique blood properties that speed the process of thrombosis.
All these factors can be due to surgery, air travel, obesity, pregnancy, prolonged bed rests, cancers, burns, changes of hormonal balance in blood, genetic factors such as plasminogen disorders as well as factors associated with lifestyle (Righini 2010). The actual diagnosis of PE in patients is achieved through laboratory tests and clinical assessment of the symptoms exhibited by the patient. Normally the assessment of the clinical symptoms is the preliminary diagnosis that is usually undertaken which should form the basis for a more detailed diagnosis which involves medical imaging that we shall discuss more comprehensively in the following sections.
In clinical assessment of symptoms a guideline that was developed by Wells et al to simplify and streamline the physical diagnosis of cases is routinely used which is referred to as the Wells Score (Righini 2010). These are diagnoses that usually involve assessment of a patient in different key areas which are assigned numerical scores that would be used to determine the initial diagnosis of PE. This diagnosis is usually confirmed through more detailed and specific tests; usually the CT and echo scan. In the case study scenario, the patient exhibited the classical symptoms of PE which included unconsciousness, difficulty in breathing, and cardiopulmonary arrest which led to the diagnosis of Pulmonary Embolism.
Patient Preparation and Contrast Protocols
CT angiography relies on a computer tomography software application that enables it to generate an image of pulmonary arteries that indicate whether embolism exists. Iodinated element is injected into the blood material in order to provide the necessary contrast that is required to highlight the artery lumen. The nitroglycerin element in iodine is responsible for the vasodilatory and luminescence effect that enables the CT imaging to be possible (Silber 1990).
This laboratory approach of diagnosing PE using CT angiography is contraindicated to patients that are allergic or hypersensitive to iodine or the related medications of diagnosis. Other patients that are not recommended to undergo CT angiography include patients with thromboembolic disorder, patients with history of heart failure, hyperthyroidism, attrial fibrillation and pheochromocytoma (Hoffman, Ferencik, Cury & Pena 2006).
The CT angiography scan requires patients to be dressed in loose and comfortable clothing, such as gowns without any other dressings such as belts, metal hearing aids or dental metal works since metal objects affects the CT image quality.
For best results, the patients are also not required to have eaten or drank water few hours before the CT scan examination and pregnant patients are advised not to breastfeed for the next 24 hours.
This is because of the side effects that the iodinated chemical that is used to give contrast can cause to the unborn through the breast milk; the reason it works best in patients that have not drunk or eaten anything prior to the scan is due to the rapid absorption rate of the agent in the blood. (RadiologyInfo.Org, n.d.). During the CT scan, the patient are laid on the examination table in a supine position and injected with 70-100ml contrast agent through an intravenous line that has a flow rate of ideally 5mL/s (CTisis.com, n.d.)
Scanning Protocol of CTA
The CT angiography involves three important steps; topogram, initiation of CT angiography and the CT scan itself (Raff, Gallagher & O’Neill 2005). During the first stage the topogram is obtained which allows the process to proceed to the next step which is initiation of the imaging process of the CT scan. The second stage ensures that the blocked areas of the arteries are enhanced by the contrast agent that is administered through the timing bolus technique or bolus tracking technique.
Bolus tracking technique requires several dose axial scans that are done at intervals for purposes of tracking bolus as well as monitoring the enhancements levels in arteries to make sure they do not exceed the 100 Hounsfield units. The timing bolus technique on the other hand applies a single bolus of iodinated agent together with saline that is injected later in the antecubital vein in order to determine the duration of time required by the contrast. Regardless of the technique that is used the results should be similar.
The last step that completes the preparation of CT angiography scan is a CT volume dataset of the coronial artery; the scan is then taken on a single breadth hold with high concentration of iodine injection that is set to flow under high pressure.
The quality of CTA scanning is a factor of several key characteristics that include collimation, reconstruction spacing, pitch and scan timing when applying injection (Raff, Gallagher & O’Neill 2005). This quality is additionally affected by low quality z-axis resolution which is normally used in CT scanners that is responsible for poor resolutions in 3d reconstructions. Hence, the key is to optimize the output of the z-axis resolution which can be enhanced by well structured reconstruction protocols and CTA scanning. This is where the variables of pitch, slice thickness, collimation and reconstruction interval comes in. Optimal determination of collimation and pitch levels are mostly determined by three factors; patient characteristics, nature of venous access and region of examination (Medscape.com 2000).
The challenge here is to determine the most appropriate levels for both pitch and collimation in consideration of these factors, this is because when collimation is narrow and pitch is set low, the z-axis resolution is enhanced but inadvertently increases probability of tube heating and scan time. So as a rule of thumb pitch should not exceed 2 units; when faced with a choice of either increasing collimation or pitch for large scans the decision should always to opt increasing the pitch up to this maximum limit (Medscape.com 2000).
The standard protocol for CTA reconstructed slice thickness is 0.8 mm where the image increment is set at 0.4 mm, ideally this is for a pitch factor of between 0.2 and 0.3 subject to other factors. Besides CT imaging, ECG-gated CT can be used to diagnose PE in patients, this is because ECG gated technique is able to detect and visualize blood motions in arteries caused by heart beats.
This means that the principle of ECG-gated that is applied in cardiac CT imaging can also be extended to enable visualization of the abdominal region albeit with some form of modification on the technique. The technical requirements of the CT scan is that it should have 16 slice scanner as it minimum, but higher slice scanners are preferred since they provide more accurate slice collimation and improved temporal and spatial resolutions. The whole CT scan procedure from the time of patient preparation and imaging processing usually take 15 minutes on average (Raff, Gallagher & O’Neill 2005).
Radiation Exposure
Like all forms of body scans, CT scanning also inarguably exposes the patient to significant dosage of ionizing radiation. On average full body CT scan discharges as much as 12 mSv radiation while a targeted region might result to as much as 2-10 mSV, this is a risk level of approximately 0.08% in developing cancer from such an effect.
A standard 64 slice CT scanner can give out a radiation dosage that is in the range of 11-12 mSv, unfortunately dosage modulation is not possible with almost all types of CT scanners but this dosage is safe to majority of patients that have regular heart functions. In fact the dosage of radiation that is given out by such a scanner mentioned above is similar to radiation emitted by approximately 100-160 repeated posteroanterior chest radiographs (Hoffman, Ferencik, Cury & Pena 2006).
The fact that this radiation exposure is cumulative makes it all the more necessary to put in place strategies that limit the amount of radiation exposure in patients. Generally there are three factors that can modified to minimize radiation exposure in patients; time factor, distance and amount of shielding (Franklin 2002). Due to the advances made in technology the level of radiation exposure has drastically been reduced with the advent of the spiral CT machines. This is because this technology makes it possible for duration of time taken to scan a patient to be drastically reduced meaning that less exposure to radiation takes place.
Image Display, Appearance and Analysis
CT angiography is done in spatial mode; the quality of the final imaging will therefore depend on the various data acquisition parameters. But image reconstruction specifications requires 75 mm slice thickness, 0.4mm increment and 512 by 512 pixel matrix; thinner slice than this will increase resolution and the overall image quality but at the expense of increased image noise. The problem with high image noise is that it limits the accuracy and assessment of the diagnosis since CT images are normally restructured with a medium smooth kernel. Since the CT dataset is usually large with almost 500 axial images, there is usually the challenge associated with storage of this amount of dataset.
The nature of the axial images is also a point to consider since original axial images are more reliable and therefore most likely to identify the pathological condition; nevertheless, they are still effective when combined with multiplanar reformatted images. The principle that is used to locate the position of the embolism involves identification of the arterial narrowing that is characterized by calcified and non-calcified plaques as shown on the axial images.
But this will only narrow down the location of the embolism just enough for the 2-long axis view of the artery to be blown out by the 4-7 slices that present it in one image, at this point the location has been zeroed in enough to be identified (Hoffman, Ferencik, Cury & Pena 2006). The final image that is processed from this dataset provides a general overview of the artery as indicated in the following image.
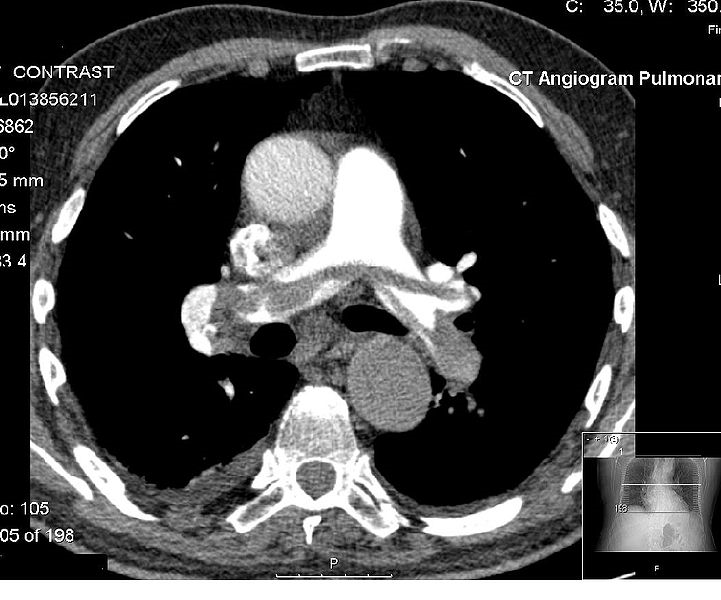
The Saddle Pulmonary Embolism that is shown in the above image was identified through the following characteristics of the CT scan angiograph; flattened or negative T wave, right ventricular dilation, sinus tachycardia and systolic pulmonary. Other information that is necessary to complete the diagnosis includes the nature of isotropic data scan and the individual axial sections.
The image below of a CT scan can be used to explain how the 2D multiplanar display can be used to prevent misdiagnosis in the evaluation of the pulmonary vasculature, while figure 3 is an image that highlights the importance of 3D visualization. The patient on whom the images were taken exhibited symptoms of chest pains that led to suspicion of pulmonary embolism and CT scan of the pulmonary vasculature was done to confirm the diagnosis. As shown on the image, the axial section indicates intraluminal filling defects that were treated with heparin but remained unchanged.
Later diagnosis however confirmed the condition to be pulmonary artery sarcoma; a diagnosis that was enabled by the 3D display because of its ability to highlight this condition. The 3D visualization also suggests that the disease is invasive and extends towards the periphery, making PE to be an unlikely cause (Schoepf 2004).
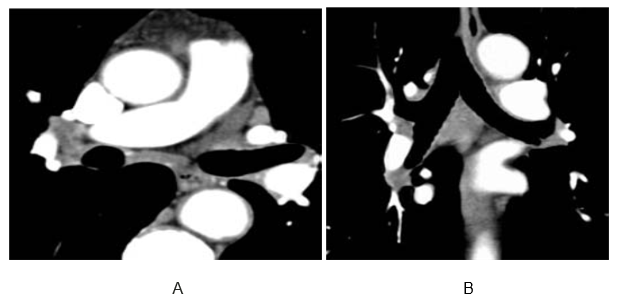
In a patient with lymphadenopathy, lymph node tissue adjacent to the pulmonary arteries can be mistaken for a thrombus and intra-arterial filling defect within the pulmonary vasculature. (A) Two-dimensional multiplanar display, (B) particularly a coronal reformat, avoids this common pitfall.
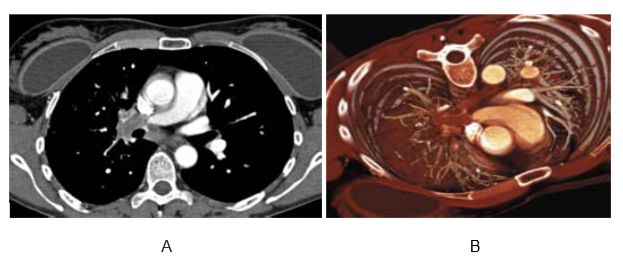
In a patient with acute chest pain, axial sections show intraluminal filling defects suggestive of pulmonary embolism. Pulmonary artery sarcoma was visualized on a three-dimensional display, which shows invasive disease extending further into the periphery than would be expected of a pulmonary embolism.
Both 2D and 3D provide valuable evaluation and assessments that enables physician to arrive at accurate diagnosis of the pulmonary condition that a patient is suffering from. More importantly these imaging techniques are important in communication of the patient pathogenesis during referral since the nature and extent of the condition can be derived from looking at the image (Schoepf 2004).
Aftercare of Patients
The care of patients that have been diagnosed with Saddle Pulmonary Embolism and treated through surgery involve careful and regular use of medication, follow up clinical visits, light exercise program that is monitored by a physician and a total ban on smoking. It is necessary to give blood thinners to some patients that have been treated for PE; in this case attention must be given to symptoms that include nose bleeding, gum bleeding and blood in urine.
In general, patients treated blood thinners should be very keen from engaging in activities that might lead to bleeding such as dental cleaning, injuries through sport or even cuts from shaving. Finally, due to the risk of excessive bleeding that can cause death, patients that have been treated with blood thinners should carry information on themselves that indicate this fact in case of an emergency (Drugs.Com, n.d.)
Treatment and Prognosis
The standard treatment procedure for PE cases involves a combination or any of the following; surgery, anticoagulant therapy, thrombolysis and inferior vena cava filter implantation. Provision of anticoagulants is the most common method of treatment, while the most commonly used medication in PE treatment is heparin medicine, usually enoxaparin, fondaparinux and dalteparin. In low risk cases, PE treatment is usually done for a period of 3-6 months’ especially for first time cases while it can be a lifelong treatment process for patients that have repeat diagnosis of the condition.
Treatment by thrombosis requires the use of enzymes in medications that destroys clots in blood and prevents embolism. Surgical implantation of the filter vena cava inferior is usually the last option that physician turn to when the other modes of treatment are undesirable or ineffective due to it long term implications
Research findings of one study on the subject published by Barrit and Jordan showed that the rate of mortality from untreated PE was as high as 25%, while the diagnosis of the condition was influenced by variety of factors as well as the medical history of the patient (1990). Overall the research study concluded that the major risk factors for pulmonary embolism across the cases are; obesity, pregnancy, immobilization and blood conditions (Barritt & Jordan 1990).
The case study patient mentioned in this paper was finally diagnosed with type II Prothrombin, a blood condition that causes blood clots to occur at an excessive rate. The patient was treated through anticoagulant therapy and given Coumadin which is an anticoagulant to continue using after she was discharged (Chappell 2006).