Introduction
A radiopharmaceutical, otherwise known as nuclear medicine, is made up of one or more radioactive isotopes called radio-nuclides. Radiopharmaceuticals are pharmaceuticals prepared in readiness for applications in curing and/or identification of diseases in persons. Radioactive element 133Xe, 131I-NaI, or a labelled compound 131I-iodinated proteins and 99mTc-labeled compounds are a few examples of radiopharmaceuticals. There are two main components of radiopharmaceuticals which include: a radionuclide that gives the preferred radiation qualities; and verification of the in vivo spreading and physiological characteristics of the radiopharmaceutical, a specified chemical compound is required. A radionuclide, which is an unstable nucleus, possibly will decay by emitting various kinds of ionizing radiation: gamma (γ), positron (β+), beta (β-) and alpha (α) radiation (Sharp, Gemmel & Smith, 2005).
Alpha emitters, could be described as mono-energetic and display considerably a very short span in matter for reasons attributable to their mass. This has the effect of leaving almost all of its energy on a very minute area (stretching to a few cell diameters). Alpha emitters are applied only for purposes with therapeutic outcomes. Their applications in clinical medicine are very rare, and they are predominantly applied in for research and development missions. For radionuclides enriched by using neutrons, these break up and decay by emitting beta (β-) radiation. Beta emitters characterize diverse energy quantities, and have varying range in matter (from 40 to 100μm) determined by their energy. Just like the alpha emitters, Beta emitting radionuclides have primary applications in the field of therapeutic radiopharmaceuticals. Positron (β+) decay takes place in nuclei enriched by using proton (Sharp, Gemmel & Smith, 2005).
A precise work out of the time-dependent signal in body tissues is necessary for calculating the affected areas of the body absorbed dose. The applied schema, which gives key methods for calculating the taken in dose of radio-nuclides that has internally accumulated, is called Medical Internal Radiation Dose (MIRD). Whole- body retention is resolved by quantitative imaging, recovering of body excreta totally and quantitatively, non-imaging, which is observation using a probe set externally and sampling the blood directly which yields results for blood activity (Loevinger & Berman, 1976).
In order for the applied schema to work, three stages are necessary. The first stage is the accumulation of data; the second stage is the study of data; while the last stage is processing of data. This paper describes only the second phase mentioned above, that is, the study of data, which will give descriptions on techniques used in quantitative measurement. These techniques include: Techniques of Planar Imaging using Scintillation Camera, PETS and SPECTS. Non-imaging techniques are omitted altogether. Rigorous mathematical calculations are omitted but a few formulae are included (Loevinger & Berman, 1976).
Techniques of Planar Imaging using Scintillation Camera
There are several factors that influence the precision in measuring the quantity of radioactivity using a scintillation camera. The following are some of the factors: limitations on separation of energy into constituent parts; degradation of separation of space components due to collimator high-energy of photons passing through thin partitions in organism and consequences of spreading radiation; statistical noise related to low mass count and other interferences; the inherent resolution of the scintillation crystal- NaI(Tl), the Compton scatter and collimator influence the resolution of space in planar images; and geometric sensitivity (the amount of emitted photons divided by unit time which gets through to the crystal from a circumscribed angle position) depends upon the applied collimator. Examples of types of collimators are converging, pinhole and diverging and parallel-hole collimators. The approved collimators for measuring the quantity of radioactivity are parallel-hole collimators. These collimators have less geometric distortion in comparison to other types.
Scintillation camera resolution of space weakens in proportion to the increase of distance between the source and the detector. Therefore, the closer the subject to the detector is the better the spatial resolution. Data related to the activities of radiopharmaceutical doses absorbed in the whole body and identifiable localities are obtained from views of planar scintillation camera. For spread radiopharmaceuticals in a single locality or secluded non-superimposed regions in the planar projection, this method gives optimum accuracy. Majority of scintillation camera systems applied in radiopharmaceuticals are computer-based and software is available for automated or semi-automated techniques for acquisition of very complex data and carrying out statistical analysis (Thomas, Maxon & Kereiakes, 1988).
Technique of Conjugate View Counting
Conjugate view counting method is favoured by many and applied often in taking imaging measurements of radioactivity. It comprises of a combination of a system calibration factor, data transmitted through the subject and 1800 complementary planar images. This technique presents an enhancement over the single-view approach which relates to contrasting a specified phantom under predetermined geometry in that the thorough mathematical theory for conjugate view methods for measurements presents adjustment for attenuation, in-homogeneity and source thickness. Results from calculations related to tissue source depth are distinct from theoretical point of view. For situations where activities of source region are not time-dependent, a definite conjugate-view measurement taken is generally acceptable. Present day scintillation cameras with two heads offer suitable methods and efficient protocols for immediate acquisition of the two images and generally permit means for A/P scans of whole-body. However, single-head camera arrangements could be applied with repetitions of imaging and positioning as necessary to get the conjugate view. The arrangement calibration factor is necessary to translate the source region count rate into resolved activity. It is important to measure the calibration factor at each time of acquisition point to document that the arrangement response stays invariable or give explanations for any variation in performance that might have influence on the examined count rate. The pair of conjugate-view image is in general and in relation to the source region, an anterior and posterior (A/P) image. However, any real l800 opposed arrangement could be applied.
Two examples of mathematical calculation formulae are given here below
For isolated single source region the calculation is as follows.
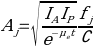
- Where Aj is source activity;
- Ia∧If are counts/time;
- e is transmission factor;
- fj is correction for the source attenuation coefficient
- μ and source thickness
- t and
- C is count rate per unit activity. For 4 overlapping source regions, the calculation is as follows.
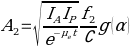
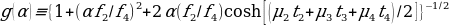
The examples given are for source regions that have background surrounding and the related activities could be ignored. There are other methods associated with rigorous calculations of conjugate view counting such as: – subtraction related to simple back grounds; analytical formalism; number pseudo-extrapolation; methods related to factors of build-up; methods of multi-energy windows; and techniques of digital filtering, among others. However, there are situations where features or volumes are shown by scintillation camera on one view only. A method applying actual point source of a single straightforward view is handy (Leichner et al., 1993).
The techniques that are applied in this field of nuclear medicine are numerous and two more approaches discussing PETS and SPECTS are highlighted below. There are backgrounds of various regions that are irregular in structure and lack homogeneous composition intrinsically. Complications are inevitable and calculations of radiopharmaceutical activities are expected to be very involving and sophisticated. Interferences make the situation even much more complicated. Actual measurements of activity concentration become a formidable task. The techniques of conjugate view counting described above cannot be applied in this scenario. This is where SPECTS come into operation. In this category we have varieties of SPECTS techniques including among others the following: SPECT systems multiple detectors; multiple cameras on ring system; fan beams; con beam; scintillation rotating camera of single type; use of filters for statistical uncertainty effects reduction and many more. Many factors connected with regions of imaging view have to be considered that affect the results accuracy. These are statistical noise, intrinsic sensitivity, spatial penetration, energy resolution, transmission attenuation, tissue densities, geometric structural formations, limitations attributed to quantitative measurements, and many more.
PET provides the most accurate techniques for calculations of radiopharmaceutical activities in situations where the above described methods are not adequate and /or where cost is not top priority. Automated computer driven systems are highly desirable in the PET domain. Hybrid types of sophisticated digital techniques are common for PET and accurate spontaneous multi dimensional calculations are carried out. Needless to say, PET is very expensive and rarely found in ordinary institutes. Top scientific research laboratories have equipment employing PET technology. PET is occasionally applied in measuring activity of a positron emitter to replicate another radionuclide of the same atomic number where by the activity measurements and absorbed dose approximations are preferred. The concept is made that isotopes have the same bio-kinetic performance. Contrasting most positron emitters, 1241has amply prolonged half-life to allow imaging over many days throughout the biologic washout and uptake of the agent. Consequently, once a comparable positron-emitting isotope that has a material half-life that is adequately long relative to the pharmacokinetics is available, PET imaging possibly could enhance the accuracy of measurements of activity (Thomas, Maxon & Kereiakes, 1988).
Conclusion
Computational methods expressed and highlighted in this paper have applied the MIRD schema for absorbed dose evaluation. Many analytical methodologies for present and future applications and likely design of experimental trials have been discussed by exhaustive consideration of information already available and distributed touching the radiopharmaceutical for assessment of the suitable number of investigational sampling positions to be acquired supported by uptake and retention attributes. It is very important to have available a correct resolution of the time-dependent activity in situ is because this is necessary for calculating absorbed dose to the concerned regions. The answers to questions of over-all activity A and residence time T entail ascertaining a probable plan for data compilation, analysis and dissemination. After categorizing all source regions with enough sequential sampling, the complete activity in every one of these regions in comparison to time should be established. Quantitative measurement methods such as conjugate view planar imaging and inclusive of SPECT and PET imaging have been presented and clearly explained by giving areas, suitability and limitations of applications. Notwithstanding, not all methods of imaging techniques have been covered. Needless to say, the scope is very wide and only the most important and in conventional use have been selected for this paper.
References
Loevinger, R. & Berman, M., 1976. Revised schema for calculating the absorbed dose from biologically distributed radionuclides, MIRD Pamphlet No. 1 Revised. New York: The Society of Nuclear Medicine.Leichner, P. et al., 1993. An overview of imaging techniques and physical aspects of treatment planning in radioimmuno therapy. Medical Physics, 20, pp. 569-577.
Sharp P. Gemmel, H. & Smith, F., 2005. Practical Nuclear Medicine. Oxford, UK: University Press.
Thomas, S. Maxon, R. & Kereiakes, J., 1988. Techniques for quantitation of in vivo radioactivity. In: Gelfand, M. & Thomas, S. (eds.). Effective use of computers in nuclear medicine. New York: McGraw-Hill.