Theoretical Background
The electrical conductivity of a solution corresponds to the solution’s ability — or, more precisely, the dissolved substance — to conduct an electric current. This phenomenon’s theoretical basis lies in the substance’s dissociation plane when dissolved in liquid media. For most substances, water molecules cause intra- and intermolecular bonds to break, creating a collection of charged ions from a single molecule (Flowers et al., 2019). The dissociation of electrolytes produces both positively charged ions (cations) and negatively charged particles (anions), with the value of their charge determined by the nature of the initial substance and the concentration determined by the initial concentration of the dissolved substance.
When an electric current is applied to a solution containing anions and cations, the ions are aligned depending on their charge. The ordered movement of charged particles in the solution under the action of an external field forms an electric bridge, which closes the chain and creates the possibility for the free flow of current. However, it is of research interest to determine the factors that affect the intensity of such current transfer. It is well known that conductivity depends on the nature of the dissolved substance, the concentration, and the temperature of the solution but is not affected by its pH and the presence of non-ionic dissolved substances (Flowers et al., 2019). Thus, the conductivity of electric current in solution is a function of many variables.
The electrolytic conductivity of a solution has a direct relationship with athletic dehydration. During intense athletic activity, the individual loses moisture through profuse sweating, and thus, a dehydration phase sets. In such a phase, there will be an excessive concentration of electrolytes in the body’s fluid media, which causes an increase in electrical conductivity. If the body does not replenish the water used up, the body can experience severe consequences of dehydration, including aerobic and cognitive dysfunction.
Research demonstrates that using sports drinks rich in electrolytes and carbohydrates to rehydrate may be more effective than drinking water (Meyer et al., 2019). Similar results have been shown in another study indicating that water consumption alone cannot effectively cover the deficits in athletic dehydration (Halder & Daw, 2020). Thus, the problem of solution conductivity related to electron concentration also has practical applications.
The concentration and nature of the soluble substances were chosen as the critical determinants of conductivity in this laboratory work. The first goal of this work was to determine the effect of increasing the concentration on the conductivity of the solution. Based on this, the first hypothesis was that an increase in electrolyte concentration, that is, an increase in the number of charged ions, led to an increase in electrical conductivity. The second goal of the work was to determine the effect of the nature of the substance on the ability to conduct. Consequently, the hypothesis for this purpose was that increasing the number of ions in the electrolyte molecule, that is, increasing the number of particles formed during dissociation, led to an increase in conductivity. Thus, the research laboratory work was designed to assess the validity of these hypotheses.
Methods
The methodological basis for the present laboratory work was the execution of the experiment. Expressly, after formulating the hypotheses and becoming familiar with the goals and procedures of the project, an installation connected to a conductivity probe was set up in LabQuest. Distilled water was added to the cylinder to confirm and calibrate the probe, and measurements demonstrated zero conductivity, confirming that the probe worked correctly. By analogy, conductivity was measured for three different solutions containing different concentrations of NaCl, CaCl2, and Ethanol. Dissolved substance drops in 50 ml of water were used as a measure of concentrations: the more drops of substance were added, the higher the concentration. A total of three starts were used, one for each dissolved substance.
Data on the dependence of the conductivity of solutions for different substances at different concentrations were collected and presented in tabular form. Statistical analysis of these data implied plotting a scatter plot with a regression line. Regression corresponds to constructing a straight line that best covers all the data in the set, and a measure of this reliability is the coefficient of determination (Zhang, 2020). The slope coefficients determining the ratio of increase in conductivity to increase in concentration were recorded for each solution and discussed in the context of the electrical conductivity of the solutions.
Results
The preliminary results of the laboratory work were the conductivity data recorded with the probe in LabQuest as a function of the number of drops of each substance added. Table 1 summarizes these data: At first glance, it is particularly noticeable that the organic substance ethanol changes its conductivity virtually unchanged with increasing concentration, in contrast to calcium chloride and sodium chloride.
Table 1: Summary of Data for Conductivity of Three Dissolved Substances as a Function of Concentration
These data were plotted on a single coordinate plane (Conductivity vs. Concentration) to explore the patterns more deeply, as shown in Figure 1. Visualizing each distribution with regression lines gives some insight into the differences in conductivity. First, the conductivity for calcium chloride increased much faster than for the other substances, as confirmed by the significance of the slope of the regression line (see Table 2). It follows that the conductivity of CaCl2 increased on average 1.6 times faster than sodium chloride and more than 4,700 times faster than organic matter.
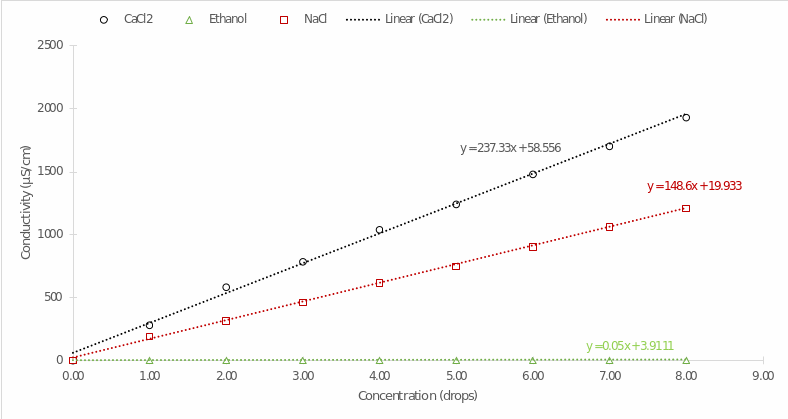
Table 2: Slope Coefficients for Each Regression Model
Discussion
In the present laboratory work, the dependence of the conductivity of the electric current for three substances of different natures as a function of their concentration was evaluated. The results demonstrated that conductivity is a function of concentration, and it can be postulated with some accuracy that there is an upward linear trend between the variables. As concentration increases, there is an increase in conductivity, as shown in Figure 1. This is not coincidental since increasing the concentration of electrolytes creates a more significant number of charged particles simultaneously in solution, and thus, there is a greater potential for conducting an electric current.
The result fully supports the first research hypothesis, and therefore, it can be assumed that all substances of electrolytic nature are characterized by this conclusion. It can be seen, however, that the conductivity of ethanol practically did not change with increasing concentration, which creates a contradiction. Although ethanol is a weakly polarized molecule, it cannot conduct electric current (Table 1) effectively, so it is not a classical electrolyte. This justifies the apparent lack of change in the conductivity dynamics of ethanol, even with increasing concentration.
In addition, it was shown that the conductivity of calcium chloride was maximal at any concentration compared to other substances (Figure 1) and had a higher value (237.33) slope (Table 2), which supports the second hypothesis of the experiment. The moderate value (148.60) of the slope was characteristic of sodium chloride, and the lowest (0.05) of organic matter of non-electrolytic nature. Since all solutions had the same initial concentration, the difference in the growth rate of conductivity may be due to the nature of the molecule. In particular, when dissociated, calcium chloride yields three particles (1 Ca2+ and 2 Cl-), sodium chloride only two (1 Na+ and 1 Cl-), and ethanol hardly dissociates in water. Thus, the dissociation of calcium chloride always yielded a larger number of charged particles, which justified the higher potential to conduct current.
Despite the support of the two research hypotheses, the work may have been associated with some inaccuracies and errors. The sources of such errors could be incorrect measurements of the conductivity of substances and errors in recording the results in the table. In addition, the regression line is only an approximation, which means that one cannot be entirely sure that a perfect linear relationship between the variables exists.
The initial calibration of the probe could also be considered a source of error because if the calibration is incorrect, the data measurement is inaccurate. During the cleaning of the probe between measurements, it could have been contaminated by environmental substances, which could also have affected the accuracy of the measurements. Nevertheless, the present laboratory work can be considered a success because it proved the relationship between current conductivity and the concentration of the dissolved substance and between conductivity and the nature of that substance.
References
Flowers, P., Langley, R., Robinson, W. R., & Theopold, K. H. (2019). Chemistry 2e. OpenStax.
Halder, S., & Daw, S. (2020). Importance of sports drinks as a performance prerequisites. Senhri Journal of Multidisciplinary Studies, 5(2), 09-19. Web.
Meyer, F., Timmons, B. W., Wilk, B., & Leites, G. T. (2019). Water: Hydration and sports drink. In D. Bagchi, S. Nair, & C. K. Sen (Eds.), Nutrition and enhanced sports performance (pp. 545-554). Academic Press.
Zhang, D. (2020). Coefficients of determination for generalized linear mixed models [PDF document]. Web.