Introduction
A double-stranded DNA molecule copies itself to create two identical DNA molecules through the intricate process of DNA replication. All organisms follow this essential process, which guarantees the preservation of genetic information. A method or technique may instantly replicate billions of items—roughly 100 billion—if certain criteria are met.
Protein synthesis is the mechanism by which genetic information is expressed. DNA replication is required during cell division in order to ensure proper genetic information or DNA transfer to the two new daughter cells. Because they concurrently synthesize the leading and lagging strands of DNA at the replication fork, enzymes are essential to DNA replication. This essay will analyze different enzymes, outlining their roles in DNA replication and their significance. From a biochemical perspective, enzymes’ role in DNA replication is vital, as these catalysts facilitate and regulate the complicated process of duplicating genetic material.
Enzymes Involved in DNA Replication
The intricate process of DNA replication entails copying a cell’s whole genome. During various phases of DNA replication, distinct enzymes are essential. The double-stranded DNA helix is unwound by the first enzyme, DNA helicase, resulting in a replication break where the strands may be replicated. By breaking hydrogen bonds between nitrogen base pairs during the process of DNA “unwinding,” helicases speed up replication divisions (Brosh & Matson, 2020). Simultaneously, the individual DNA strands close to the replication site are wrapped around by specialized proteins called single-strand binding proteins, which stop them from re-associating and creating a double helix. It could be simpler for other enzymes to connect with the exposed DNA strands during replication.
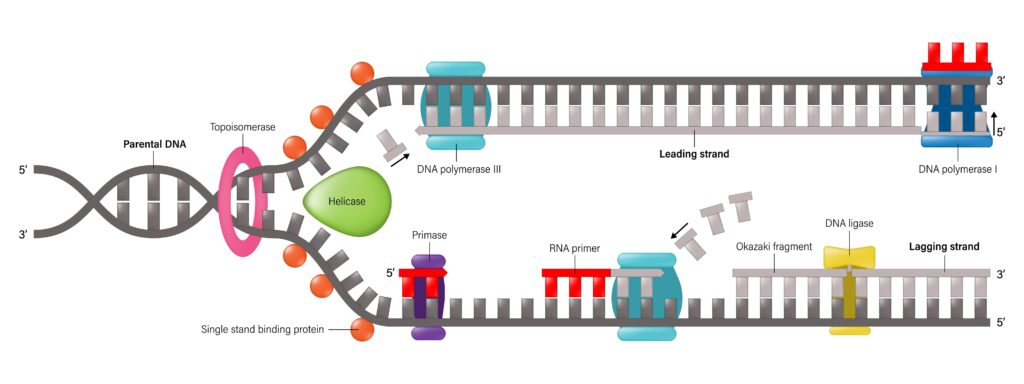
Figure 1 illustrates how DNA helicases catalyze the breaking of hydrogen bonds that tether the two strands of double-stranded DNA (Brosh & Matson, 2020).
The tension in the DNA strands caused by the helicase’s unwinding is released by the following enzyme: DNA gyrase (topoisomerase). Replication proceeds in two distinct steps on the leading and lagging strands of DNA, with the 5′ to 3′ replication direction having the most significance (Brosh & Matson, 2020). Tension builds up in areas before separation during DNA unwinding, which is aided by DNA helicase and causes rewinding.
By lowering tension and preventing DNA breakage that may happen during the unwinding process, DNA gyrase helps with this rewinding. During transcription and replication, these enzymes aid in the folding and unwinding of DNA (Hirsch & Klostermeier, 2021). Prokaryotic gyrase and topoisomerase inhibitors interfere with the reconnecting of phosphate bonds when DNA strands fracture, limiting the introduction or decrease of supercoiling. This has an impact on DNA replication, transcription, and repair.
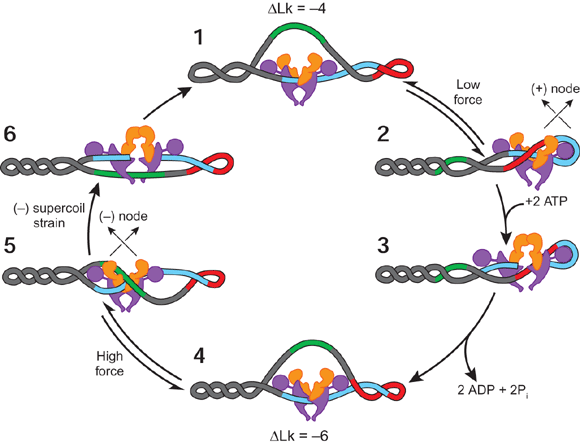
Figure 2 demonstrates how removing the C-terminal DNA-binding domain from the A subunit of gyrase results in an enzyme that cannot supercoil DNA but exhibits DNA relaxation in an ATP-dependent manner (Hirsch & Klostermeier, 2021).
Another enzyme that is utilized in DNA replication is called DNA primase, and it creates short RNA primers that are complementary to the DNA template. RNA primers that match the DNA strand are produced by primase (Guilliam & Yeeles, 2020). The majority of the new DNA is subsequently produced by DNA polymerase III extending these primers by appending nucleotides to the 3′ end.
The primary enzyme involved in DNA synthesis, it contributes nucleotides to the developing DNA strand’s 3′ end (Guilliam & Yeeles, 2020). For instance, the primary function of DNA polymerase III is to meticulously add nucleotides, one at a time, to the 3′ end of an emerging and expanding strand in order to extend a single strand of DNA. DNA polymerase I then substitutes DNA for the RNA primers. This enzyme aids in the process of replacing DNA nucleotides with RNA primers. DNA polymerase I produces brief DNA segments in vivo in eubacteria during excision repair. Furthermore, during lagging strand replication, it contributes to the removal of RNA primers and the filling of the spaces between Okazaki fragments.
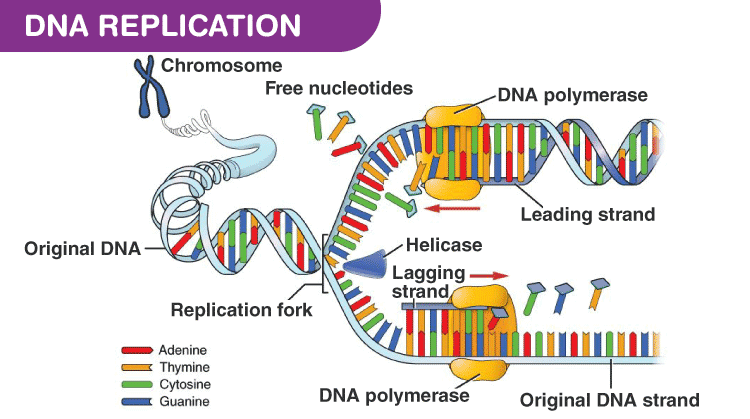
Figure 3 illustrates how DNA polymerase operates in pairs, collaborating to produce two identical DNA strands from a single original DNA molecule (Guilliam & Yeeles, 2020).
The synthesis process is finally completed when DNA ligase seals the spaces created by the broken DNA pieces. On the lagging strand, the ligase fills in the spaces left by the Okazaki fragments to form a continuous, whole DNA strand. This enzyme connects and consolidates the Okazaki fragments synthesized by DNA polymerase III by operating on the lagging strand, as the leading strand does not contain any (Sallmyr et al., 2023).
DNA ligase activity joins tiny pieces of freshly formed DNA, named Okazaki fragments after their discoverer, to generate a full and unbroken new strand of DNA. Three genes in humans, namely LIG1, LIG3, and LIG4, encode DNA ligases that are reliant on ATP (Sallmyr et al., 2023). While the three-step ligation process is carried out by comparable catalytic regions in these enzymes, their surrounding domains differ and allow them to engage with various partners.
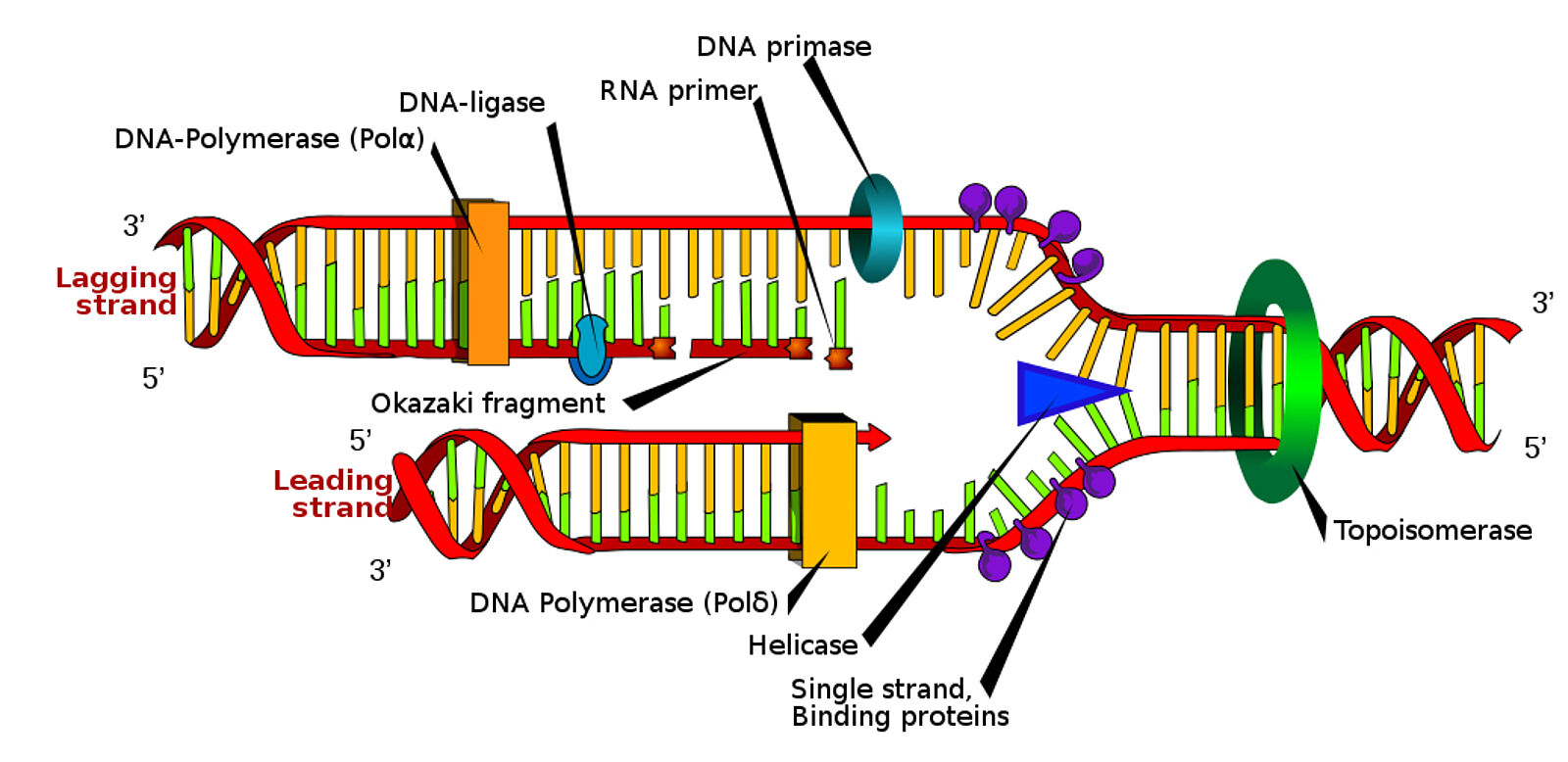
Figure 4 shows the process, during which DNA ligases catalyze the creation of phosphodiester bonds at single-strand breaks in double-stranded DNA (Sallmyr et al., 2023).
In order to stop the strands from reannealing, single-stranded binding proteins (SSBs) attach to single-stranded DNA that has been exposed by the helicase. To create new strands, it’s critical to separate the two original strands (Brosh & Matson, 2020). In order to stop the two parent strands from reannealing, single-strand binding protein, or SSB protein, binds to unpaired single-stranded DNA. The freshly synthesized DNA strand is stripped of mismatched nucleotides by exonuclease enzymes (Brosh & Matson, 2020). If the wrong base is added, the enzyme breaks the phosphodiester link and releases the wrong nucleotide. This corrective action is executed through the exonuclease activity of DNA pol III.
After the removal of the erroneous nucleotide, a replacement is subsequently added. In eukaryotes, telomerase adds repetitive nucleotide sequences (telomeres) to the ends of linear chromosomes, preventing them from shortening with each round of replication (Brosh & Matson, 2020). eukaryotic chromosomes feature specialized DNA “caps” known as composition to safeguard against the erosion of genetic material. As chromosome ends degrade, the composition varies among organisms, comprising numerous repetitions of a specific short DNA sequence.
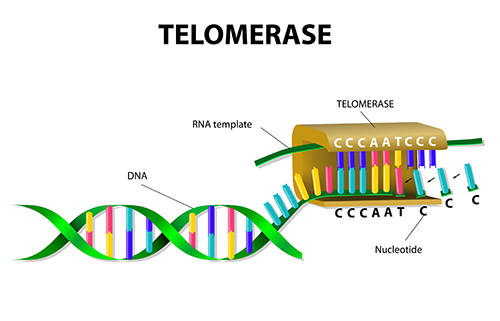
Figure 5 is an illustration of the regulation of cellular lifespan is achieved through the repression of telomerase and the induced shortening of telomeres (Brosh & Matson, 2020).
The enzymes participating in DNA replication collaborate to construct the lagging and leading DNA strands simultaneously at the replication fork. It is achieved by creating pairs of replicative DNA polymerases, each accompanied by their respective accessory proteins (Li et al., 2022). One polymerase unit synthesizes the leading strand, while the other synthesizes the lagging strand. The polymerase subunit involved in lagging strand synthesis can move in tandem with the component synthesizing the leading strand because the lagging strand template creates a loop at the replication fork.
Biochemical Significance and Regulation
Significance of DNA Replication in Cellular Functions and Inheritance
The process of DNA replication entails splitting a double-stranded DNA molecule into two identical daughter DNAs. The genetic material in the newly formed daughter cells during cell division has to mimic that of the parent cell, which makes this process essential. Each DNA strand’s capacity to serve as a template for duplication is the foundation of the replication mechanism (Li et al., 2022). It starts at certain locations, known as origins when the double helix of DNA starts to unravel. Small RNA segments known as primers act as the building blocks for the creation of DNA.
After that, nucleotides from the template strand are restored by DNA polymerase, starting the replication process. Short RNA primers complementary to the template DNA strands are produced by the enzyme primase. The next step is to eliminate these RNA primers once DNA polymerase has used them as starting points to synthesize new DNA strands (Guilliam & Yeeles, 2020). Cells reduce the possibility of introducing mistakes or mutations during DNA replication by combining repair mechanisms after replication with proofreading during replication. The cell can divide into two identical copies of itself after DNA replication is finished.
Regulation and Control Mechanisms Involved in DNA Replication
The cell’s ultimate objective is to split into two compartments, each containing an identical copy of the genome. The genomic replication technique must thus be precise. Errors that cause the genome to be under or over-replicated throughout any cell cycle can have detrimental effects on human health, including cancer, birth deformities, and a variety of developmental problems (Wilhelm et al., 2022). Cell cycle checkpoints are examples of molecular regulatory mechanisms that are intended to guarantee that the genome replicates just once and is then equally distributed among the ensuing daughter cells.
Replication machinery, known as the replisome, is directed to certain chromosomal regions known as origins in order to control DNA replication. A molecular device called the replisome replicates DNA from these sources in both directions semi-conservatively (Wilhelm et al., 2022). While elongation alludes to the replisome’s continued DNA replication, initiation describes the replisome’s delivery to these origins. Regulation mostly concentrates on the beginning phase and, consequently, the hiring procedure.
One step in the control stage that guarantees DNA replication starts just once in each cell cycle is licensing the replication origin. The recruitment process is started by building the pre-replicative complex, which marks the location of the upcoming recruitment of crucial replication machinery. Adjustments to the replication rate are performed at the start of the process whenever this becomes required. Embryonic cells may utilize more replication origins than somatic cells, allowing for simultaneous initiation events and faster overall replication.
Conclusion
In conclusion, the complex process of DNA replication is vital for perpetuating genetic information. It is a collaborative effort orchestrated by a diverse collection of enzymes. Such enzymes include helicases, DNA polymerases, primases, ligases, and topoisomerases. They play a crucial role in DNA’s unwinding, synthesis, repair, and structural maintenance during replication. This essay has explored different enzymes and the role they play in replication. It also sheds light on the intricate biochemical choreography that underlies DNA replication. The explanation of the role of enzymes in DNA replication from a biochemical perspective unveils the magnificence of nature’s molecular machinery, where these catalysts function harmoniously to memorialize life’s genetic heritage.
References
Brosh Jr, R. M., & Matson, S. W. (2020). History of DNA helicases. Genes, 11(3),1-45. Web.
Guilliam, T. A., & Yeeles, J. T. (2020). An updated perspective on the polymerase division of labor during eukaryotic DNA replication. Critical reviews in biochemistry and molecular biology, 55(5), 469-481. Web.
Hirsch, J., & Klostermeier, D. (2021). What makes a type IIA topoisomerase a gyrase or a Topo IV? Nucleic Acids Research, 49(11), 6027-6042. Web.
Li, X., Wang, L., Liu, X., Zheng, Z., & Kong, D. (2022). Cellular regulation and stability of DNA replication forks in eukaryotic cells. DNA repair, 120. Web.
Sallmyr, A., Bhandari, S. K., Naila, T., & Tomkinson, A. E. (2023). Mammalian DNA ligases; roles in maintaining genome integrity. Journal of Molecular Biology. Web.
Wilhelm, T., Said, M., & Naim, V. (2020). DNA replication stress and chromosomal instability: dangerous liaisons. Genes, 11(6), 1-34. Web.