Introduction
Studying the physicochemical properties of samples in the laboratory is necessary to better understand their nature and potential behavior when interacting with other substances. One such property is the force that conditions the intermolecular interaction of a sample. In general, this force should be understood as a characteristic of attraction or repulsion between molecules, and this force can depend on many factors, including the nature of the substance itself and the molecular weight (Karas et al., 2020).
In molecular weight, the larger the molecule, the stronger the intermolecular forces tend to be since large molecules have more electrons, resulting in stronger bonds between them. Unlike intramolecular bonds, which allow atoms within a structure to be held together, intermolecular bonds form the spatial configuration of bound molecules.
Intermolecular forces include Van der Waals forces and hydrogen bonds. Van der Waals forces, which include dipole-dipole interactions and dipole-induced dipole interactions, are much weaker than hydrogen forces that arise from the interaction of atoms with significantly different electronegativity values. In general, the stronger the intermolecular interaction, the stronger the particles are bound to each other, and therefore, more energy must be applied to separate them. This laboratory investigates the intermolecular interaction of three different liquids with different functional groups and chemical properties, namely water, ethanol (alcohol), and acetone (ketone).
The analysis in the laboratory work is based on an understanding of the property of evaporation of liquids in the air: evaporation reflects the process by which molecules detach from each other and pass into a gaseous state. Accordingly, the stronger the intermolecular interaction, the worse the substance lends itself to evaporation. Evaporation can be quantitatively measured by examining the temperature difference as evaporation cools the substance (LibreTexts, 2022).
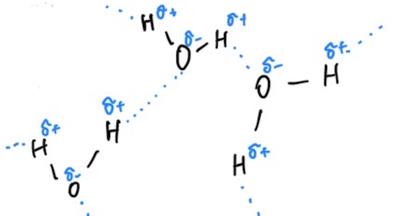
Thus, the main goals of the laboratory work were to obtain data on the temperature patterns of evaporation for each sample and to determine which one had the greatest strength of intermolecular interactions. The central hypothesis was that water, having the maximum number of hydrogen bonds due to its structure, would have the minimum temperature difference and, thus, the maximum intermolecular forces. On the contrary, acetone, having no hydrogen bonds due to the lack of suitable hydrogen, would have the maximum temperature difference and, thus, the minimum intermolecular forces.
Methods
A temperature probe wrapped in a paper towel was connected to the LabQuest and dipped into the measured liquid. Instrumental analysis specifications included a standard number of samples per second and a collection period of 240 seconds. Water, ethanol, and acetone were used as samples: the probe was placed in the sample to be studied for 45 seconds, then pulled out and held upright in the air for another 195 seconds until it reached the 240 level on the stopwatch.
The maximum and minimum temperatures were recorded for each measurement, and their difference, ∆T, was determined by subtraction. The data were recorded in Table 2 and then used for analysis. In addition, the work included building chemical structures for each sample and determining the active hydrogen that would be involved in the construction of the hydrogen bonds, which was entered in Table 1.
Results
Table 1: Structural Formulas and Descriptions of Intermolecular Interactions for the Studied Samples
Table 2: Results of Thermometric Analysis
Discussion
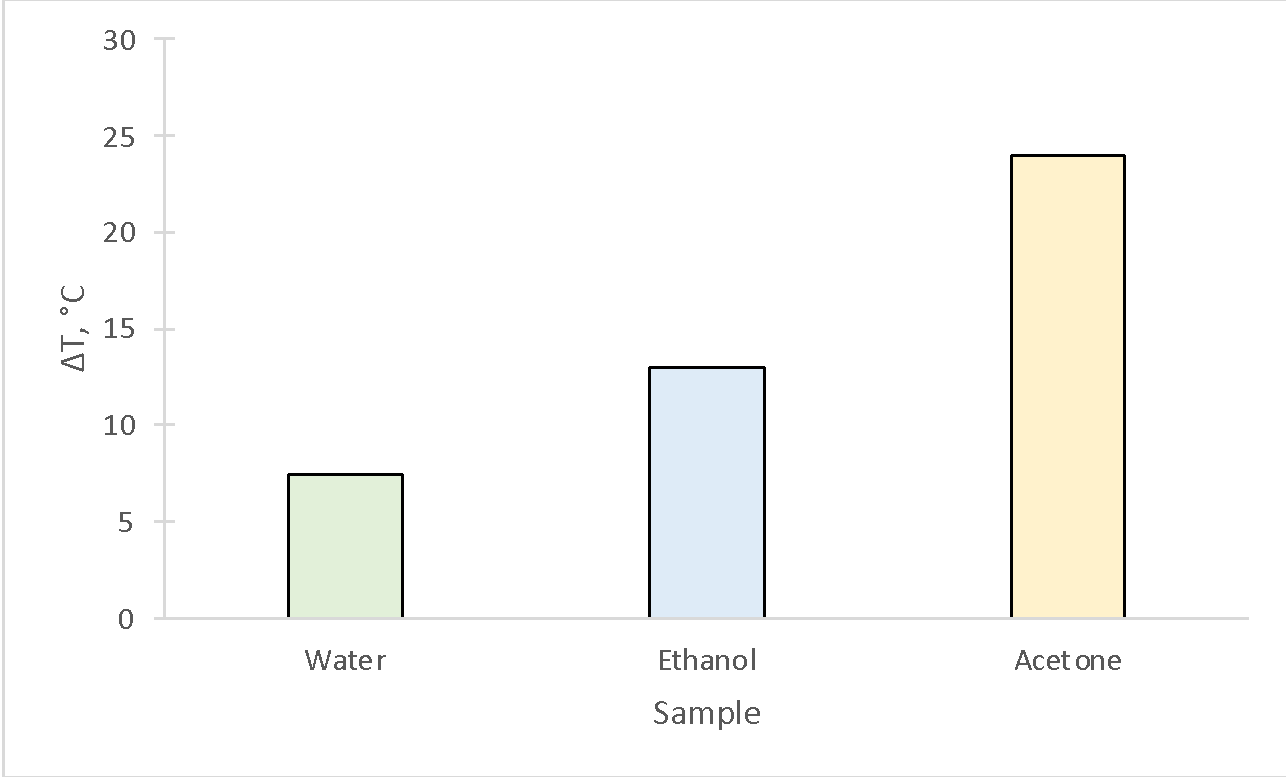
The present experiment investigated the strength of the intermolecular interactions for water, ethanol, and acetone by examining their evaporation patterns. The results demonstrated that acetone had the highest temperature difference, from which it followed that the molecules within it held together the worst (Table 2, Fig. 1). In other words, the intermolecular interactions in acetone were the worst realized. On the contrary, the stronger intermolecular interactions were characteristic of ethanol and even more so of water.
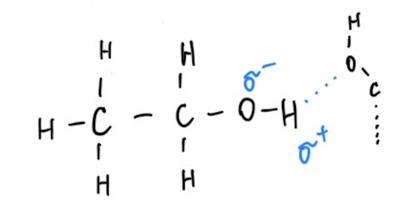
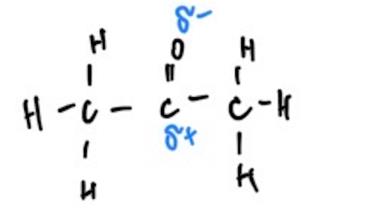
The difference in the intensity of intermolecular interactions can be explained by the substances’ polarity and ability to form hydrogen bonds (Table 1). The phenomenon is that water is a polar molecule because it has partial negative and positive charges at different ends. As a result, water molecules can form hydrogen bonds, and each molecule can form up to four hydrogen bonds (Karas et al., 2020; LibreTexts, 2019). Unlike water, ethanol can only form three hydrogen bonds because it has a non-polar hydrocarbon tail, which means that the molecules’ holding power between each other is lower (Clark, 2023). At the same time, acetone does not have hydrogen bonds to more electronegative atoms, so it cannot form hydrogen bonds (Wu et al., 2019).
At the same time, acetone can form Van der Waals forces via dipole-dipole momentum, but these forces are significantly weaker than hydrogen. It is the ability to form them, as well as their quantity, which determines the intensity of intermolecular bonds for the substance. In other words, the nature of the molecules themselves was the factor that determined water to be the substance with the strongest hydrogen bonds and, thus, the slightest temperature difference. Both hypotheses were confirmed, which made it possible to relate practical results to theoretical expectations.
Despite the experiment’s success, the work may have been associated with some errors and inaccuracies. The primary source of error could have been the observation itself since the temperature probe after each sample could have been at different levels with different air mass temperatures, which could have influenced the evaporation rate. In addition, the possibility of instrumental error should not be excluded if, during the experiment, the probe distorted the results due to technical breakdowns. It is also worth considering that during the 12 minutes of data collection, the air in the laboratory room could have changed its temperature, which could also lead to changes in the evaporation rate of the samples.
The most significant temperature difference corresponded to the condition in which the molecules were better detached from each other, which means that the intermolecular interaction was weakest. This connection is because when a liquid evaporates, sufficient kinetic energy is transferred to the molecules, allowing them to break off and transform more easily. This process requires energy, which is taken from the internal energy of the liquid and leads to the cooling of the liquid. The results showed good agreement with theoretical expectations, so the goal of the work was achieved.
References
Clark, J. (2023). Hydrogen bonding. LibreTexts Chemistry. Web.
Karas, L. J., Wu, C. H., Das, R., & Wu, J. I. C. (2020). Hydrogen bond design principles. Wiley Interdisciplinary Reviews: Computational Molecular Science, 10(6), 1-41. Web.
LibreTexts. (2019). Hydrogen bonding. LibreTexts Chemistry. Web.
LibreTexts. (2022). Phase changes. LibreTexts Chemistry. Web.
Wu, N., Li, X., Liu, S., Zhang, M., & Ouyang, S. (2019). Effect of hydrogen bonding on the surface tension properties of binary mixture (acetone-water) by Raman spectroscopy. Applied Sciences, 9(6), 1-10. Web.