Experimental Procedure
Gravimetric analysis is used as a laboratory chemical technique to identify, for example, the empirical formula of a substance or to utilize stoichiometric calculations through a series of successive heating and weighing of a compound that includes water molecules. In the present work, gravimetric analysis was used to evaluate stoichiometric patterns in reactions with copper(II) sulfate pentahydrate. For this purpose, several chemical processes resulting in the formation of CuO were initially carried out. In particular, an excessive amount of 6M sodium hydroxide was added to a solution containing divalent copper ions, resulting in the formation of a precipitate. Heating the precipitate filtered with paper initiated the decomposition of the substance into water and copper(II) oxide. The filtered CuO solid was analytically weighed, and the percentage yield of the substance in the processes carried out was calculated from the weighing results.
Observation
For two successive reactions, changes in the signs of the reactions carried out were observed. On the interaction of excess alkali with divalent copper ions, the formation of a solid precipitating on the bottom was observed to be blue in color — this was copper(II) hydroxide, Cu(OH)2. Heating the filtered blue solid produced colorless water vapor and a black solid, copper(II) oxide, CuO. No other visual changes were observed in the reactions taking place.
Results and Discussion
Two sequential chemical processes were involved in the present experiment, the balanced equations of which are given below:
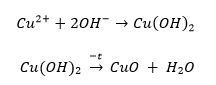
As can be seen from the equations shown stoichiometrically, the number of moles of copper ions and copper(II) hydroxide in the first reaction are equivalent. For the second reaction, similar numbers of moles for copper(II) hydroxide and copper(II) oxide are also observed. It follows that the total number of moles of divalent copper ions appears to be identical to the mole content of the black precipitate of copper(II) oxide. The initial mass of copper(II) sulfate pentahydrate, the source of copper ions, was 2.301 g, which was converted to moles:
moles (Cu2+) = 2.301 g * 1 mol/249.685 g = 0.0092 moles
As shown above, a similar number of moles was relevant for CuO, which leads to the calculation of the theoretical mass of the black precipitate:
mass (CuO) = 0.0092 moles * 79.55 g/mol = 0.73g
Given that the actual mass of CuO obtained on the analytical scale was 0.712 g, this gives the solution for the percent yield:
yield (%) = 0.712 / 0.730 * 100% = 97.1%
Conclusion
The present work was aimed at describing the results of laboratory gravimetric analysis used to study the stoichiometric patterns of substances or compounds in running processes. The report showed that the experiment involved two equations, namely the interaction of divalent copper ions with excess hydroxide ions to form a blue precipitate and the subsequent heating of the precipitate to form black copper(II) oxide and water. In terms of stoichiometry, it was shown that the total number of moles of copper ions from copper(II) sulfate pentahydrate was identical to that for CuO.
Considering that the initial mass of copper(II) sulfate pentahydrate was known, and also considering the results of analytical weighing of CuO, the theoretical mass of CuO was first calculated for this report, and then the percent yield was determined. The results showed that the yield of these processes was 97.1%, indicating that the laboratory work was extremely accurate: only 2.9% of the substance was lost.
Some problems may have been present when performing the analytical work. Firstly, it cannot be ruled out that not all of the substance was transferred from the reaction dishes to the scales, which could have reduced the final mass of the substance and thus led to a drop in the percentage yield. Secondly, it cannot be ruled out that there were random errors that could have led to inaccurate results — to eliminate these, it would be prudent to use a retesting strategy to reduce potential measurement and calculation errors.