Introduction
Iodine is an essential trace element found in nature that is critical for human well-being. It exists in several chemical forms, including the stable ion iodide, as well as iodate, hypoiodite, and the diatomic molecule. Because of its nature, researchers have designed various metal oxide materials for capturing and storing it (Muhire et al., 2022). People get Iodine from sources like iodized salt, dairy, and some grains. Failing to get enough Iodine (deficiency) can cause serious health issues, such as goiters, hypothyroidism, and cretinism (Eales, 2019).
Iodine metabolism in humans involves the ingestion and absorption of Iodine from the diet, its transport to the thyroid gland, and the subsequent production and release of the thyroid hormones triiodothyronine (T3) and thyroxine (T4) (Eales, 2019). The measurement of Iodine in the human body is an essential factor in assessing overall human health. Various methods exist to measure iodine levels, including the 24-hour urinary iodine excretion (24UIE) test, serum iodine test, thyroid test, peroxidase antibody test, and hair analysis. Understanding the importance of Iodine in the environment and its potential applications is significant for human health research.
Iodine in the Environment
Iodine is a trace element found in the environment in the form of iodine-containing compounds, such as Iodide (I−), iodate (IO3−), and hypoiodite (IO−). According to Zhang et al. (2019), Iodine is also found in its elemental form, existing as diatomic molecules (I2). In the environment, elemental Iodine is generally unstable, as it is highly reactive and quickly forms more stable compounds (Zhang et al., 2019).
Iodide (I−) is the most common form of Iodine found in the environment. It is highly soluble and is readily absorbed by plants and animals, making it a valuable dietary source. Iodide is also one of the most stable forms of Iodine and is resistant to oxidation and transformation by microorganisms (Muhire et al., 2022).
Iodate (IO3−) is the environment’s second most common form of Iodine. It is slightly less stable than Iodide and is more likely to react with other elements and compounds to form more stable forms. Hypoiodite (IO−) is the environment’s least common form of Iodine (Muhire et al., 2022). It is highly reactive and can quickly transform into more stable forms, such as Iodide.
To capture, store, and reuse Iodine in the environment, researchers have developed a variety of materials based on metal oxides. These substances, including metal-organic frameworks and bismuth-based compounds, have the capacity to capture, store, and release Iodine in a regulated manner (Reda et al., 2021). For instance, Reda et al. (2021) investigated the use of bismuth-based materials for the collection and storage of Iodine. In contrast, Muhire et al. (2022) investigated the use of metal oxide-based materials. Both researchers acknowledged that solution chemistry plays a significant role in the release of Iodine from iodoacetate in aqueous environments (Muhire et al., 2022; Reda et al., 2021).
Sources of Iodine
Iodine can be found in various sources, including iodized salt, sea vegetables, dairy products, certain grains, and iodized water. The amount of Iodine in each source can vary depending on the specific product and region. For instance, Farebrother et al. (2019) stated that iodized salt typically contains 45-60 mg of Iodine per kg, while sea vegetables can range from 25 to 1,000 mg.
Dairy products also vary, but generally contain between 10 and 60 mg/kg of Iodine (Baccarini et al., 2020). Furthermore, Baccarini et al. (2020) established that some grains can contain up to 4 mg of Iodine per kg, while iodized water can have up to 1.2 mg of Iodine per liter. In this case, Iodine can be found in diverse sources as indicated above.
Iodine Deficiency
Iodine deficiency (ID) is a condition caused by not having enough Iodine in the body. It is caused by a lack of dietary Iodine, a lack of access to iodized salt, or an impaired ability to absorb dietary Iodine from food (Machamba et al., 2021). According to Machamba et al. (2021), ID is the leading global cause of preventable mental hindrances and brain damage in children. It is also responsible for some other health problems, including goiters, hypothyroidism, and cretinism (Machamba et al., 2021). Therefore, addressing ID is an important initiative in public health disease prevention.
Iodine is a nutrient that must be included in the diet to maintain good health. Iodine is essential for the body to produce thyroid hormones, which are crucial for maintaining a healthy metabolism, growth, and development. Moreover, it is necessary for the development of red blood cells and the healthy operation of the nervous system (Hay et al., 2019). According to Hay et al. (2019), the World Health Organization (WHO) recommends that 100 to 150 g/day of iodine intake is the minimum amount required to prevent ID. Iodine intake needs to be between 250 and 300 g/day at the greatest level to avoid ID (Hay et al., 2019).
The consequences of ID can range from mild to severe. Mild ID can lead to reduced mental and physical development, decreased work capacity, and increased susceptibility to infections. Severe ID can lead to cretinism, which is marked by growth retardation, mental retardation, deaf mutism, and motor impairment (Machamba et al., 2021). In this case, further research is needed to develop initiatives for preventing ID in humans.
Iodine Metabolism
Iodine metabolism in the human digestive system is a complicated process. According to Eales (2019), Iodine metabolism involves ingesting and absorbing Iodine from food, transporting it to the thyroid gland, and producing and releasing the thyroid hormones triiodothyronine (T3) and thyroxine (T4). This makes it a crucial trace element for humans, as it is necessary for the production of thyroid hormones, which are essential for regulating thermogenesis, metabolic rate, and numerous other fundamental bodily processes.
Iodine is absorbed by the digestive system and then transported through the bloodstream to the thyroid gland. It is oxidized in the thyroid gland to produce the active thyroid hormones T3 and T4. The more active version is T3, while the more stable form is T4. The enzyme thyroid peroxidase oxidizes Iodine to produce T3 and T4, and two iodinated tyrosine molecules are then coupled (Sorrenti et al., 2021). In this case, the hypothalamic-pituitary-thyroid axis controls the metabolism of Iodine, while the thyroid-stimulating hormone controls the synthesis of T3 and T4 (TSH). The following diagram (see Figure 1) illustrates the process of iodine metabolism in the human body.
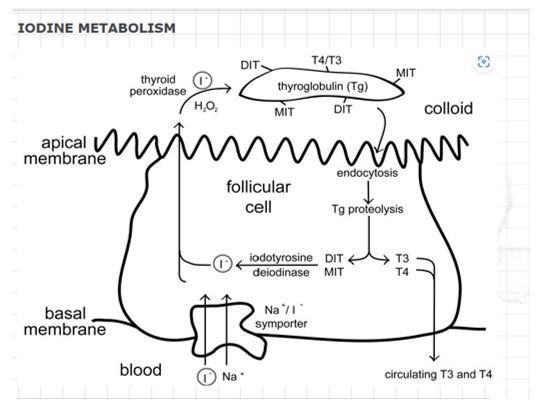
The thyroid gland produces the hormones T3 and T4, which are then released into the circulation. Studies by Eales (2019) demonstrate that once the T3 and T4 hormones are produced in the thyroid gland, they are released into the bloodstream, where they are distributed to various target tissues and organs, including the brain, liver, and muscles. Here, they act as messengers that regulate metabolic rate and other vital functions (Eales, 2019). Excess Iodine is excreted from the body via urine and feces.
Iodine as Biomarker
Iodine levels in the body are a crucial consideration when evaluating general health. Iodine is a mineral that is needed for metabolism and the synthesis of thyroid hormones. There are several approaches for calculating iodine levels, each with its own pros and cons.
24UIE Test
This reliable and non-invasive test requires only a 24-hour urine collection from the patient, which is then analyzed for iodine content (Novo et al., 2019).
The pros under this test procedure include:
- Only a 24-hour urine collection is required.
- No blood or tissue samples are taken, making it a safer alternative to other tests.
- The test accurately measures the amount of Iodine that has been excreted from the body in 24 hours.
Cons of this test include:
- The test requires a 24-hour collection period, which can be inconvenient for some patients.
- The cost of the test may be prohibitive for some patients.
- Dietary habits, drug use, and other medical conditions can all affect the accuracy of the test.
Serum Iodine Test
The level of Iodine in someone’s blood can be determined by a laboratory test called a serum iodine test. The mineral iodine is important for the synthesis of thyroid hormones. An iodine deficiency can result in hypothyroidism and other health problems. The accuracy of the serum iodine test is generally good (Abeyrathne et al., 2021). Laboratory technicians can accurately measure the amount of Iodine in the blood sample. The test results are usually available within a few days.
The pros of the serum iodine test:
- It is a relatively simple and non-invasive test that can be performed in a lab setting.
- It is also relatively inexpensive and can provide quick results.
The cons of the serum iodine test:
- It does not provide information about the body’s iodine stores, which can only be determined with a more expensive and invasive test.
- The test may not be accurate in people with certain health conditions, such as kidney failure (Abeyrathne et al., 2021).
Thyroid Peroxidase Antibody Test
The TPO Ab can also determine iodine levels. It is a dependable and precise test that can identify the number of antibodies the immune system has created in response to thyroid peroxidase (Abeyrathne et al., 2021). These antibodies have the potential to disrupt the thyroid gland’s normal function, leading to thyroid conditions such as hypothyroidism.
Pros:
- The TPO Ab test is a highly accurate and reliable test for measuring TPO antibodies in the body.
- It can detect the presence of antibodies that disrupt the normal functioning of the thyroid (Abeyrathne et al., 2021).
- The results of the test can help diagnose and monitor various thyroid disorders, such as hypothyroidism (Abeyrathne et al., 2021).
Cons:
- The test cannot measure the actual amount of Iodine in the body, only the presence of antibodies (Abeyrathne et al., 2021).
- It may produce false-positive results in people with normal thyroid hormone levels (Abeyrathne et al., 2021).
- It may also produce false-negative results in people with thyroid disorders (Abeyrathne et al., 2021).
- The test is expensive and may not be covered by some health insurance plans.
Hair Analysis
Hair analysis is a test to measure the amount of Iodine in the human body. This method of testing is often used to diagnose iodine deficiency, which can lead to a variety of medical issues, such as thyroid problems, fatigue, and weight gain (Novo et al., 2019). According to Novo et al. (2019), the accuracy of hair analysis is generally good, with most studies indicating that it can detect iodine levels with high precision.
However, there are a few potential drawbacks to this testing method. For instance, hair analysis is not able to detect iodine levels in the bloodstream and thus is not a reliable indicator of total body iodine levels (Novo et al., 2019). Additionally, hair analysis can be affected by the presence of certain medications, as well as the use of certain shampoos and styling products.
The pros of hair analysis include:
- It is simple.
- Non-invasive nature.
- Relatively low cost.
- It can provide helpful information about iodine levels that can inform treatment decisions.
The cons of hair analysis include:
- It is inaccurate in detecting total body iodine levels.
- It can be affected by other external factors (Novo et al., 2019).
- This test is unable to detect other vital minerals and vitamins, which could be important indicators of health, and is therefore inaccurate.
Methods Used to Determine Total Iodine and Iodine Speciation
Iodine can be measured using various instruments. The choice of instrument depends on the type of sample and the intended application.
Capillary Electrophoresis (CE)
CE is a technique used to separate molecules in a mixture based on their differences in electrophoretic mobility. The sample is injected into a capillary filled with an electrolyte solution, and an electric field is applied to the capillary. Separation of the sample’s molecules is achieved according to their electrophoretic mobility (Li, 2019). The concentration of Iodine in the sample can then be determined by measuring the concentration of the iodine ions in the elution profile.
Ion Chromatography (IC)
Ion chromatography is a technique used to separate ions in a mixture based on their charge. The sample is injected into a column containing an ion-exchange resin. An electric field is then applied to the column, and the ions in the sample are separated according to their charge (Ito et al., 2019). The amount of Iodine present is quantified by analyzing the concentration of its ions shown in the elution profile.
Gas Chromatography (GC)
The GC method achieves separation by exploiting the varying volatility of the molecules within a mixture. The sample is injected into a column filled with a stationary phase, and a carrier gas is used to move the molecules through the column. The molecules in the sample are then separated according to their volatilities (Xie et al., 2019). The Iodine concentration can then be determined by measuring the concentration of its ions in the resulting elution profile.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
ICP-MS is a technique used to measure the concentration of elements in a sample. To measure the concentration of Iodine in a sample, the sample is vaporized and ionized in an inductively coupled plasma source (Clases & Gonzalez de Vega, 2022). The ions are then separated and detected by a mass spectrometer (Li, 2019). The final step is to determine the sample’s Iodine concentration based on the measured concentration of iodine ions within the elution profile.
High-Performance Liquid Chromatography (HPLC)
HPLC is an analytical method that separates components of a mixture based on their varying attraction to a solid material (the stationary phase) packed inside a column. A liquid (mobile phase) is used to push the sample through this column. As Cui et al. (2019) describe, this technique is employed, for example, to quantify Iodine by injecting a sample, separating the molecules, and then measuring the concentration of Iodine as it exits the column in the elution profile.
Conclusion
Iodine is a critical trace element that exists in different forms, with Iodide being the most stable and iodate and hypoiodite being less stable. Its primary biological function is to enable the production of thyroid hormones, a process regulated by the hypothalamic-pituitary-thyroid axis. Despite its availability in iodized salt, dairy, grain, and sea vegetables, iodine deficiency is still the world’s most significant preventable cause of intellectual disability and brain damage in children.
Determining a person’s Iodine status is vital for health assessment, and many techniques are employed for this purpose. Specialized tools, such as CE, IC, GS, ICP-MS, and HPLC, are instrumental in measuring both the total iodine content and its specific speciation. This focus on understanding Iodine’s functions in the environment and the body allows researchers to better leverage its potential applications down the road.
References
Abeyrathne, E. D. N. S., Nam, K., & Ahn, D. U. (2021). Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants, 10(10), 1587. Web.
Baccarini, A., Karlsson, L., Dommen, J., Duplessis, P., Vüllers, J., Brooks, I. M., Saiz-Lopez, A., Salter, M., Tjernström, M., Baltensperger, U., Zieger, P., & Schmale, J. (2020). Frequent new particle formation over the high Arctic pack ice by enhanced iodine emissions. Nature Communications, 11(1), 1-11. Web.
Clases, D., & Gonzalez de Vega, R. (2022). Facets of ICP-MS and their potential in the Medical Sciences—part 1: Fundamentals, stand-alone and hyphenated techniques. Analytical and Bioanalytical Chemistry, 414(25), 7337–7361. Web.
Cui, W., Hou, H., Chen, J., Yu, X., Guo, Y., Tao, Z., Deng, T., Chen, Y., & Belzile, N. (2019). The speciation analysis of iodate and iodide in high salt brine by high-performance liquid chromatography and inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry, 34(7), 1374-1379. Web.
Eales, J. G. (2019). The relationship between ingested thyroid hormones, thyroid homeostasis, and iodine metabolism in humans and teleost fish. General and Comparative Endocrinology, 280(1), 62–72. Web.
Farebrother, J., Zimmermann, M. B., & Andersson, M. (2019). Excess iodine intake: Sources, assessment, and effects on thyroid function. Annals of the New York Academy of Sciences, 1446(1), 44–65. Web.
Hay, I., Hynes, K. L., & Burgess, J. R. (2019). Mild-to-moderate gestational iodine deficiency processing disorder. Nutrients, 11(9), 1-20. Web.
Ito, K., Takeda, K., & Hirokawa, T. (2019). Determination of trace iodine in seawater: Use of ion chromatography and capillary zone electrophoresis. Bunseki Kagaku Journal of Japanese Society for Analytical Chemistry, 68(4), 227-239. Web.
Li, S. (2019). Combination of chemical derivatization and high-performance liquid chromatography coupled with inductively coupled plasma-(tandem) mass spectrometry (HPLC-ICP-MS/(MS)) for metabolite profiling of medical drugs (Publication No. 8628108) [Doctoral dissertation]. Ghent University. Faculty of Sciences.
Machamba, A. A. L., Azevedo, F. M., Candido, A. C., Macedo, M. D. S., Priotre, S. E., & Franceschini, S. D. C. C. (2021). Assessment of the Impact of Salt Iodization Programs on Urinary Iodine Concentrations and Goitre Rates: A Systematic Review. Journal of Nutrition and Metabolism, 8(1), 1-7. Web.
Muhire, C., Reda, A. T., Zhang, D., Xu, X., & Cui, C. (2022). An overview on metal oxide-based materials for iodine capture and storage. Chemical Engineering Journal, 431(1). Web.
Novo, D. L. R., Mello, J. E., Rondan, F. S., Henn, A. S., Mello, P. A., & Mesko, M. F. (2019). Bromine and iodine determination in human saliva: Challenges in the development of an accurate method. Talanta, 191(1), 415-421. Web.
Reda, A. T., Pan, M., Zhang, D., & Xu, X. (2021). Bismuth-based materials for iodine capture and storage: A review. Journal of Environmental Chemical Engineering, 9(4). Web.
Sorrenti, S., Baldini, E., Pironi, D., Lauro, A., D’Orazi, V., Tartaglia, F., Tripodi, D., Lori, E., Gagliardi, F., Praticò, M., Illuminati, G., D’Andrea, V., Palumbo, P., & Ulisse, S. (2021). Iodine: Its role in thyroid hormone biosynthesis and beyond. Nutrients, 13(12), 4469. Web.
Xie, W. Q., Yu, K. X., & Gong, Y. X. (2019). Determination of iodate in iodized edible salt based on a headspace gas chromatographic technique. Journal of Chromatography A, 1584(1), 187-191. Web.
Zhang, Z., Gustin, L., Xie, W., Lian, J., Valsaraj, K. T., & Wang, J. (2019). Effect of solution chemistry on the iodine release from iodoapatite in aqueous environments. Journal of Nuclear Materials, 525(1), 161–170. Web.