Introduction
Accurate identification of an unknown substance’s composition is one of the cornerstone tasks of chemical analysis, therefore the range of potential techniques used for this purpose varies greatly. One useful qualitative determination practice is to measure the melting point for a substance since this characteristic is considered unique. Thus, by pinpointing the melting range for the sample being analyzed and comparing the data obtained with the literature, it becomes possible to identify the compound accurately.
A powerful related tool used in this study is the fact that the melting point of a mixture of different substances is lower than the boiling points of the individual components. This phenomenon is primarily due to the more active interaction of foreign molecules when heated and hence a general lowering of the necessary temperature thresholds (Nichols, 2020). It follows that a mixture of an unknown sample with a putative substance will not lead to a change in the temperature range, which means that this is an excellent way to examine the hypothesis. In this laboratory work, there is the study of melting points of both substances with known composition and their mixture, and unknown substances, followed by their qualitative identification.
Experimental Procedure
The laboratory study was formally divided into two consecutive sections: analysis of substances with established chemical composition and determination of the melting point for an unknown sample. A small amount of two known substances were ground to a powdery state, then placed in a thin-walled narrow capillary to reach the 2-4 mm mark. Thermal examinations of the two samples were conducted in two phases using the Mel-Temp apparatus: a gross temperature rise (about 10 ℃ per minute) to determine the approximate melting point and a finer, pinpoint rise (up to 3 ℃ per minute) to obtain analytically accurate data. In each case, the signal that the necessary limits of the desired interval had been reached was the first drop of liquid on the solid sample and complete liquefaction of the material.
After determining the individual melting points for the two substances, a thermal analysis was performed for their crushed mixture, and the temperature range data were compared with theoretical information about the effect of the presence of impurities on melting. The same procedures were used to identify the composition of the unknown compound, but after a separate analysis, three putative candidates were added in parallel, and melting temperatures were also obtained for the mixture. A critical analysis was then performed for the data obtained to determine the most appropriate composition.
Results and Discussion
Compounds with a known chemical composition in advance were analyzed for Benzoin and Maleic Acid. According to the reference melting point values given in the instructions for these organic compounds, Benzoin has a melting temperature range of 136-137 ℃, and exactly the same data are characteristic of the acid. Consequently, it was assumed before starting the laboratory tests that mixing the two components into a homogeneous powdery mixture should result in a lower overall melting point due to a more active interaction of molecules of different nature. The results of the primary analysis of the individual components and their mixture are shown in Table 1.
Table 1: Determined melting points for two organic substances and their homogeneous mixture using Mel-Temp.
As can be seen from the data given in Table 1, the calculated melting temperature values generally satisfied the range given in the literature, which means that it was reasonable to conclude that this method is effective for identifying the substance. Moreover, the assumption of an overall lowering of the temperature range was confirmed, as the melting temperature of the mixture of components was on average 5% lower than the original values for the individual substances.
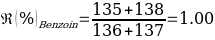
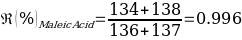
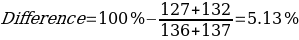
The second important study was the qualitative determination of the composition of the unknown compound, which was prepared in advance by the instructor. Among the proposed samples, the material with the numerical designation “5” was chosen. Measurement of its melting point on a Mel-Temp instrument allowed us to narrow down the search for possible candidates among the known compositions, resulting in a further study for Biphenyl, Phenyl Benzoate, and Benzhydrol. The melting data of all four compounds are summarized in Table 2.
Table 2: The data on melting points for the unknown substance and the three most probable candidates are summarized.
Based on the data obtained, a relevant assumption was made that the substance with unknown composition is Benzhydrol, whose melting point more than others matched the data obtained. In particular, the upper limit of the range is a critical parameter corresponding to the transition of the substance from solid to liquid state and thus breaking the thermodynamic equilibrium (Helmenstine, 2019). Thus, in case the substance had too small an upper bound of the temperature range, it could mean a mismatch with an unknown substance. On the other hand, the discrepancy between the average temperature of compound “5” and Benzhydrol did not exceed 1%, which indicated high analytical accuracy of the results obtained.
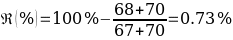
Conclusion
As a result of this laboratory work, it was shown that the use of the Mel-Temp melting point measurement method has high efficiency for the qualitative detection of the substance composition. As part of the work, it was confirmed that a mixture of two substances had a lower melting point in total than the constituents individually: the discrepancy was on average 5%. In addition, the composition of the unknown component was identified through comparison with the pool of known substances. Thus, based on the results obtained and the melting point theory, the compound with an order number “5” is Benzhydrol.
References
Helmenstine, A. M. (2019). Melting point definition in chemistry. ThougthCo. Web.
Nichols, L. (2020). Melting point theory. Chemistry. Web.