For the current assignment, the sucrose molecule was chosen for study because the compound has enormous industrial and biological potential. Sucrose is used not only as part of the artificial sugar critically used in the food industry but also as one of the most essential sources of glucose, a fundamental molecule in metabolic processes in the body. Sucrose is one of the most abundant carbohydrates in nature, found in almost all plants — for this reason, artificial sucrose is not synthesized in the laboratory but is isolated as an extract from the most abundant sources (Grover, 2022). Thus, the study of sucrose in terms of course outcomes offers a detailed analysis of a molecule of broad significance and allows us to connect the theoretical concepts studied with practical applications in a case study.
As follows from Fig. 1, sucrose is a cyclic disaccharide consisting of one α-glucose residue and one β-fructose residue connected by a glycosidic bond. This fact is confirmed by the aqueous hydrolysis of the substance, which results in the formation of glucose and fructose molecules (Peng et al., 2021). It can be seen that in the covalent bonds, the electron density is concentrated predominantly in oxygen, which is confirmed by the higher electronegative properties of this element. Due to its origin from the aqueous phases of monosaccharides, sucrose does not have a linear structure, and in the spatial context, it represents two oxygen-linked cyclic molecules arranged almost perpendicularly to each other. For the sucrose molecule, whose molecular mass is 342.30 g mol-1 and whose compound has no ionic bonds, the melting point is not large, which confirms the relationship between melting and the number of bonds in the molecule. In particular, sucrose melts at 185.85 °C, converting to caramel, which is due to the elimination of excess water, breaking intermolecular bonds and thus compacting their packing (Grover, 2022). Under normal conditions, sucrose is an excellent soluble (203.9 g/100 mL) solid in water, which justifies the high enthalpy of combustion of the substance, namely 5647 kJ mol-1. Due to the C1 (for glucose) and C2 (for fructose) positions taken in the glycosidic bond formation, sucrose does not form polymers, so the crystal lattice of the substance is molecular, where each node contains a whole molecule.
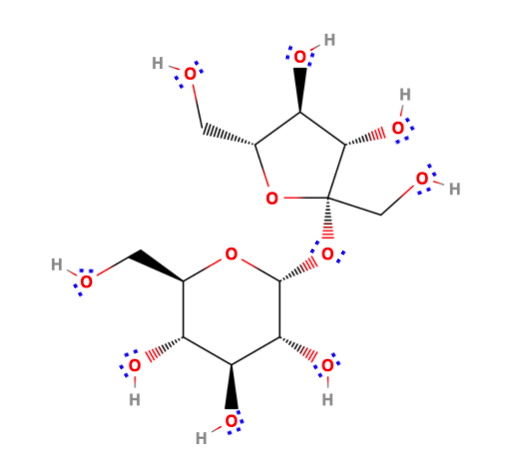
Due to the widespread use of sucrose in the domestic industry and its recognized importance to the body, the functions of the molecule continue to be explored in academic discourse. In particular, Geidl-Flueck et al. (2021) evaluated the effect of sucrose in beverages on the development of hepatic lipogenesis. In this randomized controlled trial on 94 healthy men, the effects of sucrose, fructose, and glucose on respondents’ metabolic changes were determined using a blood indicator test. It was shown that consumption of drinks rich in sucrose but not glucose led to a twofold increase in hepatic secretion and, thus, increased triglyceride synthesis. The low-density lipoproteins formed during liver function are stored as adipose tissue in the body, leading to obesity in the patient and associated metabolic problems. This article shows that consuming sucrose as one of the most popular carbohydrates in excess leads to a number of severe health problems, which is not the case for glucose. Based on these findings, nutritionists can adjust their patients’ diets to avoid the development of pathologies.
Bibliography
Geidl-Flueck B, Hochuli M, Németh Á, Eberl A, Derron N, Köfeler HC, Tappy L, Berneis K, Spinas GA, Gerber PA. 2021. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J. Hepatol. 75(1): 46-54.
Grover J. 2022. Sucrose: definition, structure, properties, uses & sample questions. Collegedunia. Web.
Peng Z, Zhou Z, Li T, Jiang M, Li C, Qing T, Yang L, Zhang X. 2021. Real-time monitoring of the sucrose hydrolysis process based on two-photon coincidence measurements. Biomed. Opt. Express 12(10): 6590-6600.