Background
The instrumental analysis method in a chemical laboratory allows stoichiometrically checking the ratios between reactants, determining the purity of the products, and predicting the course of a chemical reaction. In the present laboratory work, the organic synthesis of 1-bromobutane from 1-butanol as a feedstock is carried out on a reflux unit (Figure 1). Sulfuric acid is a medium-forming agent, and NaBr is a source of bromide ions acting as a nucleophile. This reaction is based on the nucleophilic substitution mechanism of SN2 (Ashenhurst, 2023).
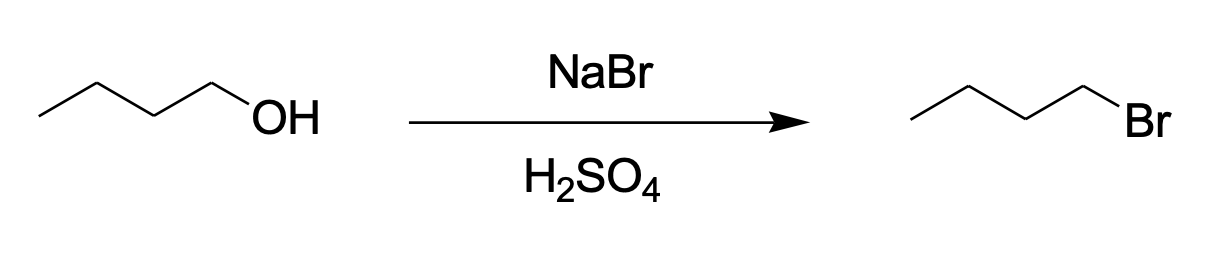
In the first step of SN2 substitution, one sulfuric acid molecule dissociates into two protons (H+) and a sulfate anion. The free protons initiate this reaction and are the medium-forming components of the mixture. The action of one proton on the 1-butanol molecule results in its attachment to the main chain, which forms a positively charged butanoxonium ion. At the same time, sodium bromide present in the medium dissociates into sodium cations and bromide anions, and the latter acts as a nucleophile attacking the butanoxonium ion.
The intermediate particle that forms includes both the hydroxyl group ions and the newly attached bromide ions. At this stage, there is an almost instantaneous reorganization of the atoms in the intermediate cation, which breaks the bond with the hydroxyl atoms and leaves only the bond with the bromide ions. The detached hydroxyl group bonds with a free proton, synthesizing the water molecule.
A distinctive feature of this organic synthesis is the use of a reflux unit. Reflux provides a stable, elevated temperature and prevents impurity product loss. This is realized by heating the reaction mixture with condensate and collecting and returning the reaction components to the mixture. In addition, a silver test is used to verify the purity of the resulting product.
The interaction of halides with silver nitrate gives a visible colored precipitate. If such a precipitate is formed, the resulting product contains halogen ions. As a negative control, however, the interaction of silver nitrate with 1-butanol is used, which gives no visible evidence of a reaction. The purpose of this laboratory report is to describe the experiment’s results and provide conclusions regarding the product obtained.
Procedure
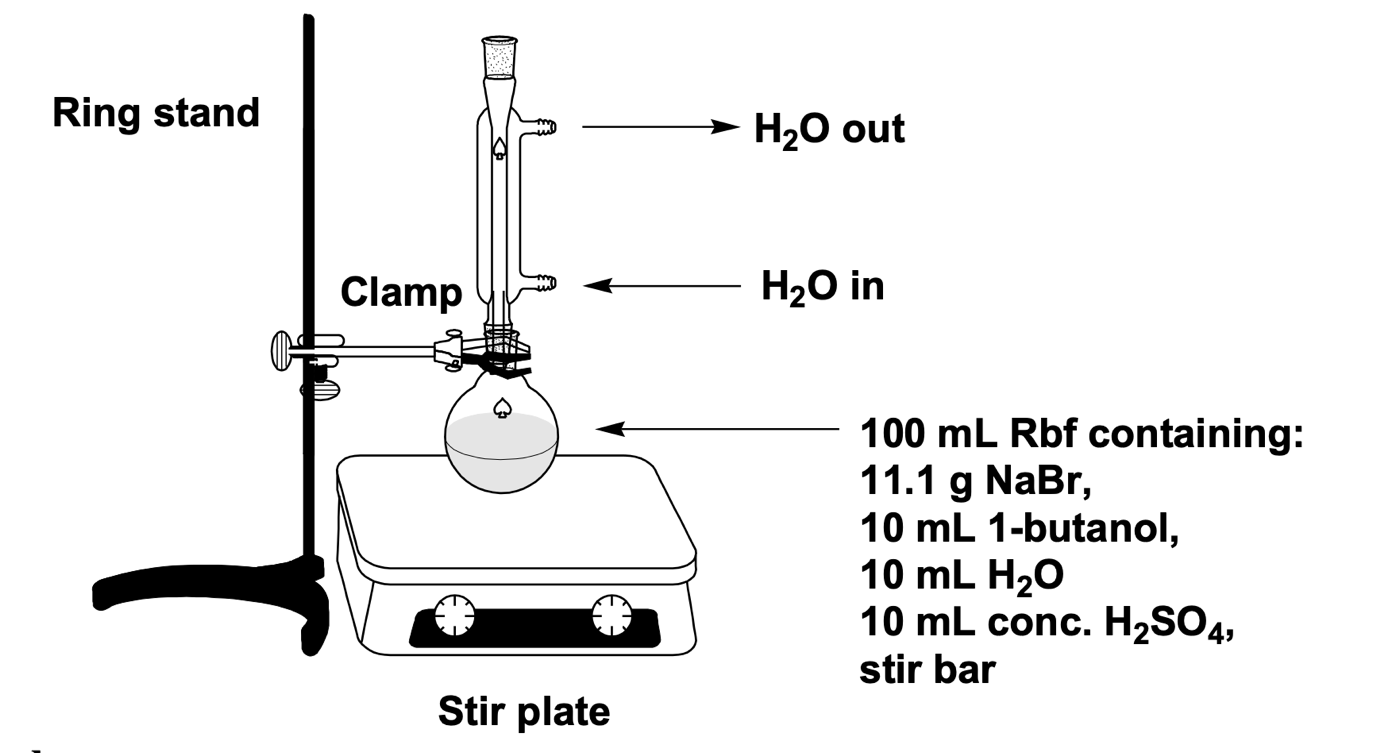
This experiment placed 11.12 g of sodium bromide, 10 mL of distilled water, and 10 mL of butanol-1 in a 100-mL round-bottom flask. After stirring vigorously with a magnetic stirrer for 1-2 minutes, the mixture was placed in an ice bath for five minutes. While the mixture was cooling in the bath, 10 mL (in 2 mL steps) of concentrated sulfuric acid was carefully added, with the mixture cooled after each step to inhibit the intense exothermic reaction.
The mixture was placed under reflux (Figure 2) and heated under constant stirring for 30 min after condensate formation. After the set time, the mixture was removed from the reflux and cooled. The mixture was separated on a separatory funnel into aqueous and organic layers several times; the organic layer was subjected to extraction in 10 mL of cold 2M NaOH, followed by 10 mL of distilled water and 10 mL of saturated sodium chloride solution.
The entire 1-bromobutane was transferred to an Erlenmeyer flask, to which six spatula tips of anhydrous sodium sulfate were additionally added. The mixture was stirred vigorously for two minutes until it became clear. The entire 1-bromobutane was transferred to a pre-weighed 100-mL flask, and the mass of the sample was recorded. The silver test was used to establish the qualitative nature of the alkyl halide obtained.
In this test, one drop of alkyl halide was added to 2 mL of 0.1M silver nitrate solution (in 95% ethanol) in a test tube. The formation of a visible precipitate of the mixture was evidence of a positive reaction. Conversely, the absence of precipitate formation is evidence of no interaction between the reactants (Clark, 2023). Thus, the test was repeated with 2 ml of 1-butanol to establish a negative control.
Results
- Weight of the weighed beaker = 52.580 g.
- Mass of beaker + sample = 55.446 g.
- Mass of sample = 2.886 g.
The results of the direct product mass measurements obtained from the analytical scales can be seen above. As can be seen from the data, the mass of the product was 2.886 g, equivalent to 0.021 moles (M = 137.02 g.mol-1). For the synthesis, 10 mL of 1-butanol was used, which, given the liquid density of 0.81 g/mL, equals 8.1 g of the starting reactant. Considering the molar mass of the alcohol (74.123 g.mol-1), 0.109 moles were used in the reaction.
Stoichiometrically, as Figure 1 shows, 1 mol of reactant is equivalent to 1 mol of product, and hence their molar ratios should be equal. In other words, the theoretically expected yield of 1-bromobutane should have been 0.109 moles, but in practice, it was only 0.021 moles. From this, it can be concluded that the percent yield of the product was 19.27% (0.021/0.109). The silver test showed that the interaction of silver nitrate with the product produced a visible pale-yellow precipitate. In contrast, the interaction of silver nitrate with 1-butanol produced no visible result (negative control).
Discussion
This work aimed to synthesize 1-bromobutane from 1-butanol using the SN2 nucleophilic substitution mechanism. The synthesis was carried out in a reflux apparatus, which helps to maintain a stable temperature and reduce the side yield of the reaction components by returning them to the mixture. This experiment yielded 1-butanol, which was confirmed by a positive silver reaction test. However, the yield of this product was only 19.27%, which is relatively low. Despite the positive reaction of the silver test, the low percentage yield of 1-bromobutane may indicate the presence of additional impurities that increased the mass of the product.
The presence of additional unpredictable products during the organic synthesis, formed during undesirable interactions between products and reactants, could be the reason for the increase in the sample’s final mass after weighing, thus skewing the molar ratio results. Thus, the experiment can be considered successful because the product was obtained. Nevertheless, the relatively low percentage yield indicates a high error and inaccuracies in the execution, including errors related to mass measurement and potential impurity components of the product. As a further extension of the current study, it is suggested that the melting point of the obtained product be evaluated, and IR spectrometry be performed to more accurately determine the analytical purity of the obtained product.
References
Ashenhurst, J. (2023). The SN2 mechanism. MOC. Web.
Clark, J. (2023). Reaction of alkyl halides with silver nitrate. LibreTexts Chemistry. Web.