The human body is a complex and multilevel system whose components are combined into a coherent and balanced mechanism. The need for molecular oxygen defines the body as an open system, which needs the resources of the environment to maintain effective activity. More specifically, at the macro level, oxygen is consumed by the individual during breathing, when the individual takes in a large portion of air with their mouth. It has been shown that this air demand reaches the 8-liter-per-minute mark, with the air being 21% oxygen: consequently, each minute, a person consumes about 1.68 liters of gas (Dutta, 2021). Indeed, not all of this is absorbed by the lungs since most of it escapes with exhaled air. However, the portion of oxygen that is actively absorbed by the blood in the alveolar vesicles and transported through circuits to all tissues and cells is of increased research interest.
Once inside the cell, oxygen is involved in an extended network of cellular respiration, which is realized inside mitochondria. It should be emphasized that cellular respiration is an energy-generating function of all cells since it allows the energy in the form of ATP molecules by oxidation of complex carbohydrates and glucose in particular (Wakim & Grewal, 2021). It is clear that oxidation processes require the participation of oxygen, then mitochondrial cellular respiration cannot proceed without the participation of gas molecules. Moreover, cellular respiration cannot also be realized without the processes of the respiratory chain since it is the latter that makes it possible to increase the amount of ATP produced significantly.
The respiratory chain should be understood as a set of biochemical processes that result in the transfer of hydrogen atoms — more specifically, protons and electrons — from coenzymatic substrates to an oxygen molecule. The coenzymes of the respiratory chain are considered to be NADH, H, and FADH2, which themselves are formed during the transfer of hydrogen protons. During this process, the reduction of oxygen is observed as the O2 molecule is converted into the water during hydrogen transfer. It is noteworthy that this water is called metabolic or endogenous because it does not come from outside but is formed naturally by the reactions of the respiratory chain (Metabolic water, 2021). At the same time, the reduction of oxygen releases a certain amount of energy, which restores the body’s resources: this is what is known as oxidative phosphorylation. The energy obtained during this process is not entirely realized in the form of ATP — in fact, ATP is formed when the phosphate ion is attached to ADP — but most of it is dissipated in the form of primary heat.
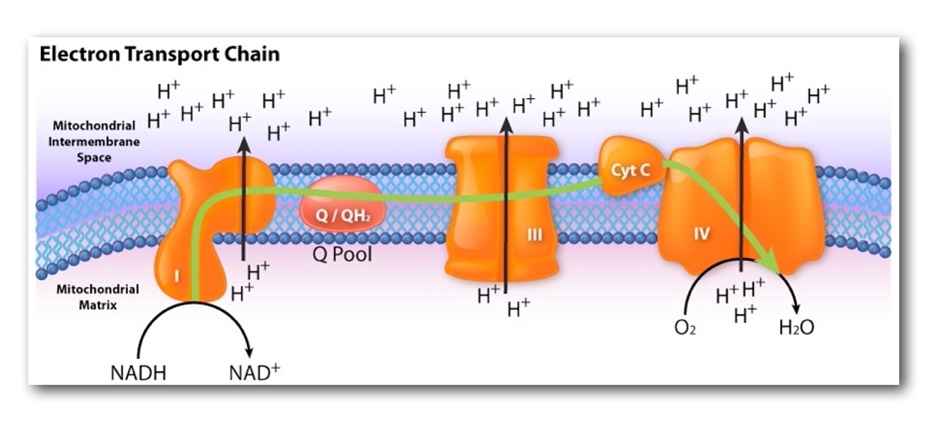
The oxygen reduction reaction described was not chosen by chance because it is this step of cellular respiration that requires the most significant amount of oxygen. In general, the entire respiratory chain requires the participation of special enzymes that catalyze the processes of proton transfer to oxygen: such molecules are called oxidases or complexes. Four such complexes are distinguished in the respiratory chain, depending on the nature of the process and the stage of the whole respiration, but complex IV is of the most significant interest within the framework of the current analysis. The last phase enzyme, or cytochrome oxidase, carries out the described process of electron transfer to the oxygen molecule, as a result of which oxygen acquires a negative charge and is converted into water molecules. Notably, cytochrome oxidase needs not only iron ions — like the other complexes — but also copper ions to accomplish this transfer, and therefore a balanced concentration of these metals in the body is critical.
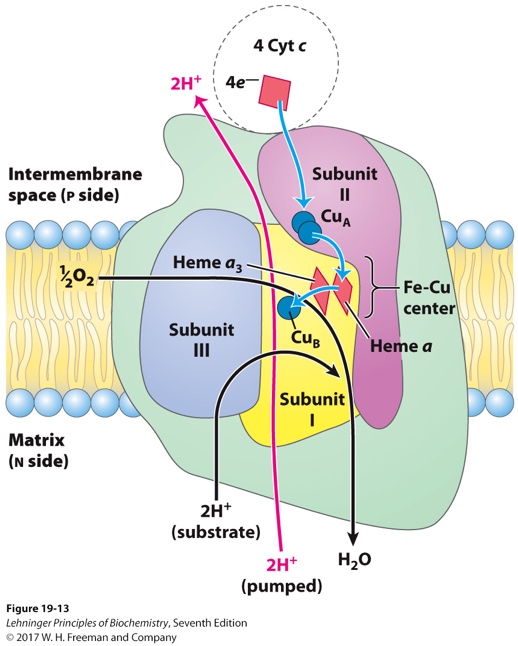
As a result, during the reading of the question for the current task, the student did consume oxygen, and this consumption was realized on the macro and micro levels. The macro-level was responsible for the penetration of gas molecules during inhalation into the lungs and the flow of gas exchange processes in the alveolar vesicles. However, the greatest amount of oxygen was involved on the microlevel, which is every second in the mitochondrial membrane. There the processes of cellular respiration and the electron-transport chain are realized, as a result of which oxygen is reduced to water molecules and energy in the form of ATP molecules is formed. This process requires enzymatic catalysts called complexes. The last of these, complex IV or cytochrome oxidase, carries out the final process of transferring four electrons to oxygen and is therefore responsible for the complete reduction of oxygen.
References
Dutta, P. K. (2021). How much oxygen a Covid-19 patient needs? Is oxygen concentrator enough? IT. Web.
Metabolic water. (2021). Biology online. Web.
Wakim, S. & Grewal, M. (2021). Cellular respiration. Libretexts. Web.