Abstract
This work aimed to obtain Phenacetin from Acetaminophen using a nucleophilic attack mechanism. Reflux, ice-bath cooling and heating, and vacuum filtration procedures were used to complete the synthesis. The simpler materials were reacted to produce complex compounds presented in later sections of the paper. Melting point values were instrumentally measured for the final product, and Thin-Layer Chromatography (TLC) and Infrared (IR) and Nuclear Magnetic Resonance (NMR) spectroscopy were performed to determine the analytical purity of the substance.
The TLC plate was used to measure the composition of the organic solvents and compounds. The plate on chromatographic principles, it has a mobile and stationary phases where solvents or their mixtures climb up the plate. The mobile phase is comprised of solvents or their corresponding mixtures, while the stationary phase is made up of the late. The percentage yield of the product was 84.3%, which indicates that most of the substance was obtained, and the experiment can be considered a success. The percentage yield was established from the analytical computations of the respective substances.
Reactions
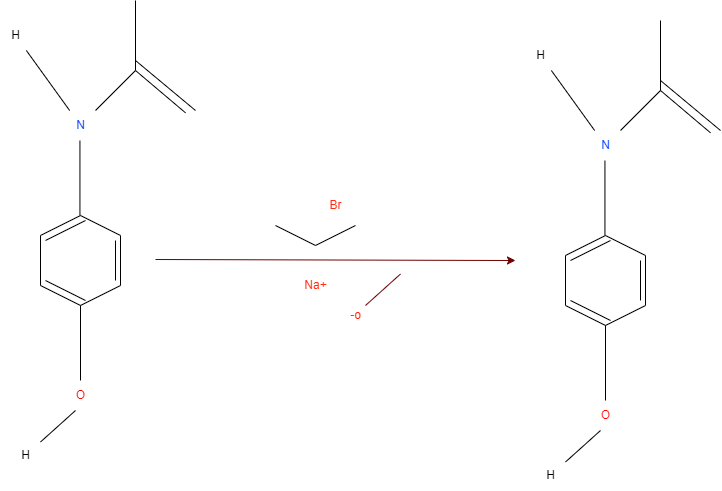
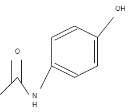
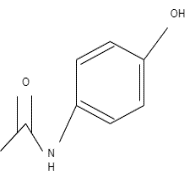
General scheme of the process of formation of Phenacetin from Acetaminophen using primary halide in the Williamson Ether Synthesis reaction.
The reaction mechanism is based on removing a proton from the hydroxyl group of Acetaminophen with the NaOCH3 participation (see Figure below). The excess electron density on oxygen attacks the alpha-carbon at the bromoethane by the nucleophilic mechanism, which leads to the displacement of NaBr and the formation of Phenacetin.
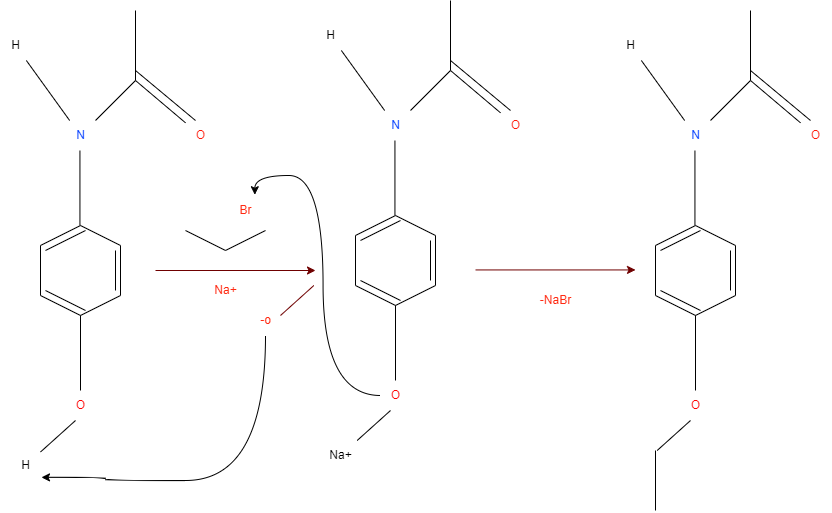
The arrows show the electron density transfers that motivate the course of this reaction.
Chemical and Physical Properties
Procedure
The performance of this laboratory work was consistent with the methodological guidelines outlined in Sneden’s textbook. This experiment is based on the organic synthesis mechanism of Phenacetin, a drug used for antipyretic and analgesic effects. Selected reagents were used for this experiment to study the organic characteristics of the final compounds. For this purpose, Acetaminophen (1.5 g) was placed in a 25-mL round-bottom flask entirely in a water bath; stirring was performed using a magnetic stirrer. The stirring was meant to ensure the Acetaminophen was uniformly heated and maintained its temperature.
Sodium hydroxide (4 mL) dissolved in ethyl alcohol was mixed with Acetaminophen and stirred intensively to achieve a uniform reaction. A reflux device was attached to the flask at 150 °C for fifteen minutes, resulting in an amber coloration of the mixture; the mixture was cooled in a water bath until the liquid phase solidified. The main purpose of water baths in this case, was to maintain the temperature of reagents and compounds at a certain temperature though heating or cooling as required during the respective stages of the reactions. Then, pre-prepared bromoethane (0.75 mL) was added to the mixture dropwise, after which second reflux was performed. After cooling, the solid part of the substance was collected by vacuum filtration. This ensures a “pure” product was collected at the end of the filtration apparatus.
In addition, a TLC plate with three points horizontally 1 cm from the bottom edge was used for the solution. On the first point, pure Acetaminophen was applied, on the second, the reaction mixture, and the third was used later. The results of the different reaction phases were recorded independently as required. The solid particles were scrubbed after vacuum filtration, added to 4 mL of ethanol in an Erlenmeyer flask, and heated at 100-200 °C, resulting in a clear light brown color. The purpose of the heating was to catalyze the reaction between the two reagents. After successive addition of 5 mL of water, the color of the solution changed to murky orange, and after heating to light peachy-amber. After cooling, the crystals of the substance were scrubbed, dissolved in ice water, filtered, and placed on point #3 in a TLC plate to perform chromatography.
Results
The yield percentage for Phenacetin was 84.3%, with the following stoichiometric considerations:
C8H9NO2 + NaOCH3 + CH3CH2Br → C10H13NO2 + NaBr + H2O
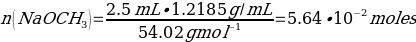
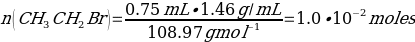
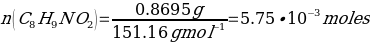
(So, this is limiting reactant)
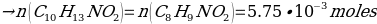
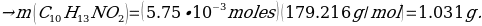
Hence, the final yield: (0.8695 g)/(1.031g.) = 84.3%
The analytical purity of the final product was essential to assess the validity of the experiment result. High purity levels imply the experiment used pure reagents, apparatus were clean and reactions carried out in the right conditions.
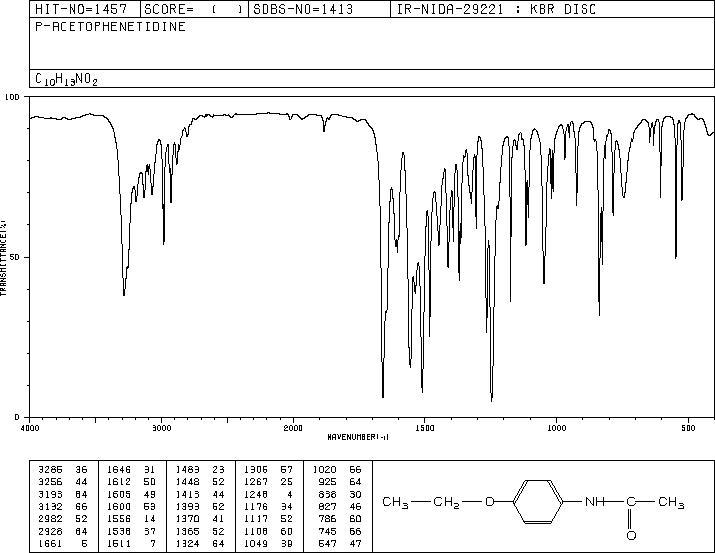
The resulting spectrogram contains the peaks for the resulting reaction product and indicates their wave numbers.

The photo contains the dynamics of product chromatographic movement and control on the thin layer.
Discussion
In the present laboratory work, Phenacetin was obtained from Acetaminophen by Williamson Ether Synthesis. It is comprised of three main functional groups namely phenyl, an ether, and an amide group. This reaction is a multi-output reaction, from which it follows that an aggregate of products may be observed at the end, which consequently affects the purity of the main product (Yearty et al., 2020). Usually, the purity of the final product is subject to the purity of the reagents used as well as the rinsing (cleanliness) of the apparatus used in the reaction. Treating a primary alcohol in the presence of sulphuric acid using a carboxylic acid produces an ester.This belief is justified by the moderate yield percentage of Phenacetin as well as the existence of impurity peaks on the IR spectrum, including the reduced intensity of the C-H carboxyl peak and N-H bonds.
The impurities in the IR spectrum are responsible for the quality of the yields obtained in the results. The reason for this aspect is the SN2 substitution mechanism in which a metal alkoxide, in this case sodium methoxide, replaces the bromine ion from the alkyl halide, which, as a result of the subsequent detachment of the sodium ion leads to the formation of a simple ester. Methyl alcohol and water, that is, proton solvents that are hydrogen bond donors, were used as solvents in this process (Diamanti et al., 2021).
IR spectroscopy was used as an analytical tool to study the purity of the product; for the same purpose, substance identification, TLC was applied. The results are a measure of the reaction of infrared radiation with mater in either of the three ways: reflection, emission or absorption. In this study, the interaction was in form of absorption and emission. The thickening of the stain at the second position indicated an increase in concentration due to the use of the reaction mixture instead of pure Acetaminophen (first position of the reaction), as well as a slight decrease in the polarity of the mixture (in the second position).
Overall, this work can be regarded as successfully accomplished, given the moderate yield percentage of the substance and the relative analytical purity. An 84.3% is can be considered a successful experiment as it implies the portion of impurities, as well as elements and compounds lost during the experiment constitute a small percentage of the experiment success. Nevertheless, the composition of the final product was not properly analyzed qualitatively, which may be a motive for improvement when the experiment is repeated. The standard physical (spotting) characteristics of are that is it is a white crystalline solid, odorless and has bitter taste. Alcohol molecules are polar and converting them to ester produces a more nonpolar compound. The validity is ascertained by the results of the TLC.
References
NIH. PubChem Compound Summary. Web.
Yearty, K. L.; Maynard, R. K.; Cortes, C. N.; Morrison, R. W. A Multioutcome Experiment for the Williamson Ether Synthesis. J. Chem. Ed. 2020, 97 (2), 578–581. Web.
Diamanti, A.; Ganase, Z.; Grant, E.; Armstrong, A.; Piccione, P.M.; Rea, A.M.; Richardson, J.; Galindo, A.; Adjiman, C.S. Mechanism, Kinetics and Selectivity of a Williamson Ether Synthesis: Elucidation Under Different Reaction Conditions. React. Chem. Eng. 2021, 6 (7), 1195-1211. Web.