Introduction
Phytoremediation is when green plants are used to treat and control hazardous chemicals and contaminants from groundwater and soil by uptaking the pollutants into the plant tissue or leaves. Examples of these contaminants are metal and metalloids, sludge, convectional wastes, and xenobiotic pollutants. The process is eco-friendly, which can mitigate various methods that remove and destroy hazardous chemicals and contaminants in the soil in a cost-effective approach (Muthusaravanan et al., 2018). There are multiple types of phytoremediation; they include phytostabilization, rhizosphere biodegradation, rhizofiltration, and phyto-volatilization.
Plants Used for Phytoremediation
Indian mustard
The plant is essential to accumulate metals and produces a large amount of biomass during the process. According to research, Indian mustard removes Cadmium from soil three times more than all plants (Muthusaravanan et al., 2018). The plant reduces Lead by 30% and Selenium by 50% (Muthusaravanan et al., 2018). Besides, it also reduces the quantity of copper, zinc, and mercury.
Willow
Willow reduces amount of diesel and heavy metals in soil through its roots. The plant reduces Cadmium and Lead quantity in the soil. Also, it is effective in mixed heavy metals like areas that have been polluted by diesel.
Poplar Tree
The critical effect of poplar trees on groundwater and soil has been researched thoroughly. They are well known for their roots which take up a large amount of water (Muthusaravanan et al., 2018). Research has shown that poplar trees can degrade hydrocarbons of petroleum, such as toluene, although the tree is not widespread in public gardens.
Indian Grass
Indian Grass is a plant which is essential for underwater and soil around them. The research has proved its detoxifying power of agrochemicals like herbicides.
Sunflower
Through sunflowers, the PAH level is reduced from the soil. Zinc, Copper, and Lead, which are heavy metals, are fed on by sunflower hence reducing their quantity in soil and groundwater.
Toxic Chemicals Removed by Water Hyacinth
Water hyacinth can absorb toxic chemicals from water, such as Strontium 90, Lead, and Mercury. Water hyacinths achieve this through their tough fibrous roots to purify water (Muthusaravanan et al., 2018). They absorb phosphorus and nitrogen, and others contaminants that toxify water. It purifies water with hazardous chemicals 10,000 times that in the surrounding.
Advantages of Water Hyacinth
Water hyacinth is used to remove agrochemical residues, like herbicides, from contaminated water. Also, hyacinth removes cyanide and arsenic from dirty water besides, the plant removes heavy metals through uptake methods.
Objectives
This lab aims to determine the rate at which water hyacinth plants remove total hardness in the form of calcium metal from a water sample.
Materials
- Water Hyacinth Plant (for $30/10 plants).
- Burette and burette stand.
- 0.01 M EDTA Titrating Solution.
- Eriochrome Black T Indicator (0.2 g in 15 mL of distilled water).
- Buffer solution.
- Calcium solution.
Experimental Procedure
- Obtain 50 mL of the initial calcium solution from the instructor (Time = 0 min sample).
- Place 2 liters of calcium solution in the aluminium container.
- Obtain a water hyacinth plant from the instructor.
- Place two of the plant in the aluminium container so that the roots are entirely underwater.
- To the Time = 0 min sample (step 1), add 1 mL of buffer solution and swirl to mix (Solution will appear wine red if calcium is present) and swirl to mix.
- Titrate slowly with 0.01M EDTA until the solution becomes blue (swirl to mix after each addition).
- Record the volume of EDTA used.
- Repeat steps 5 – 9 for solutions removed from the aluminium container at 15 min, 30 min, 60 min, 90 min, 120 min.
Results
Data Analysis
????? ???????? (??/? ?? ????3) =? ? ? ? 1000?? /?????? ????
Where:
Total hardness is calculated using the formula
Total hardness = (A×B×1000)/ mL sample used
Where A= Volume EDTA used, B=1 (in present case)
- At 0
- (14.52×1×100)/ 50 = 290.4
- At 15
- (13.72×1×100)/ 50=274.4
- At 30
- (12.74×1×100)/ 50=248.8
- At 60
- (11.18×1×100)/ 50=223.6
- At 90
- (9.94×1×100)/ 50=198.8
- At 120
- (7.34×1×100)/ 50=146.8
The total hardness of mg CaCO3 increases with an increase in the volume of EDTA used and decreased with time.
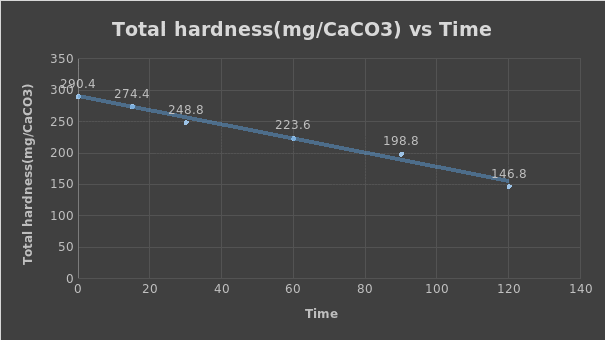
Gradient (value of slope) = change in total hardness/ change in time = (223.6-290.4)/ (60-0) = -1.113
The plotted value of the slope is -1.113, while the rate of total hardness removal is 1.113 mg / (L × min).
Conclusion
The rate at which water hyacinth plants remove total hardness was found to be -1.113 mg / (L × min); however, the value is negative, meaning the rate decreases with time. Through the experiment, one can learn the advantages of water hyacinths and plants used for phytoremediation. One can learn to calculate the rate at which water hyacinths remove total hardness from water using the gradient. The experiment was generally simple since it involved simple calculations, including addition and division, to calculate total hardness. Similarly, the graph was easy to plot and gradient simple to solve.
Reference
Muthusaravanan, S., Sivarajasekar, N., Vivek, J. S., Paramasivan, T., Naushad, M., Prakashmaran, & Al-Duaij, O. K. (2018). Phytoremediation of heavy metals: Mechanisms, methods, and enhancements. Environmental Chemistry letters, 16(4), 1339-1359.