Introduction
Shakhashiri B. Z., (1983, pp.213-215) defines a polymer as a substance composed of molecular particles branded by the numerous reappearance of one or more species of atoms or groups of atoms linked to each other in amounts appropriate to provide a set of properties that do not vary markedly with the addition or removal of one or a few of the constitutional units. Studies (Shoufeng, et al, 2001) reveal that the small molecules that react with each other to form polymer molecules are called monomers. The process of bonding that brings forms the polymer is called polymerization reaction.
One of the polymers that are formed from resins is Nylon. Nylon has the characteristic of being strong, abrasion-resistant, and mainly used in the textile industry.
The properties that arise by blending fiber and Nylon are characterized by resilience and stiffness. These give the polymer outstanding values of tranquil wash-ability, quick to dry; it is easy to press and retains its shape since it does not contract or stretch. The two common polymers are prepared by Condensation and addition. Studies by Ravez A. J., (1967), show that polymers such as Nylon 6, 10 and are usually made by the concentration of molecules and are said to be invented by Wallace Carothers in the United States. Just like most Nylon’s, the major by-product is water or HCl. The interfacial reaction of diamines and diacid chlorides and diols is normally done in two phases to produce a polyamide or nylon. Melt spinning is one way of producing Nylon which makes it available in many different forms.
Objectives
This study was set to prepare through interfacial polymerization, polyamides, and polyester and investigate the properties of both polyesters and polyamides, and document their polymer structure.
Theory
The experiment involved the reaction of 10 cm³ of in to a small beaker and adding 10 cm³ of hexamethane diamine and washing it in the sodium hydroxide solution. Immediately the polymer started forming, it was removed using a glass rod. This was repeated using sebacoyl chloride and a combination of sebacoyl chloride and adiploy chloride with careful washing in dilute alkali and water solution. The polymers were then washed using acetone and dried at 50 ºC. Since sebacoyl chloride is said to be very corrosive and reacts with water, great care was observed to prevent burns in areas of contact. Nose masks were used to regulate the amount inhaled and not eating was conducted to avoid swallowing the chemical.
To avoid the detrimental effects of severe burns to eyes, skin, and mucous membranes great care was taken while handling and hexane vapour during the experiment.
Experimental
The experiment involved making two polymers. The reagents that were used includes sebacoyl chloride (ClOC (CH2)8COCl) in hexane, hexamethylenediamine (H2N (CH2)6NH2, also called 1, 6-diaminohexane), sodium hydroxide in water, adipoyl chloride, 50 cm³ of cyclohexane, and 50 cm3 of deionised water.
The chain of bonding for Hexane-1, 6-diamine is: (H2N-CH2-CH2-CH2-CH2-CH2-CH2-NH2)
O O
‖ ‖
And that of Adipoyl chloride is: (Cl-C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-C-Cl
The apparatus used for the experiments were: One 25 cm³ beaker, Glass stirring rod, a pair of tweezers, and Retort stand with boss and clamp.
The quantities for the experiment were:
- 10 ml of 1, 6-diaminohexane (hexamethylene diamine, hexane-1, 6 – diamine, H2N (CH2)6NH2).
- 10 ml of adioyl chloride (sebacoyl chloride, ClOC(CH2)8COCl).
- 50 cm³ of cyclohexane.
- 50 cm³ of deionised water.
The Preparation
The first solution A was made by pouring 10 cm³ of adipoyl chloride in cyclohexane in a beaker and adding 100 mL of hexamethylenediamine in warm water to melt it (228°C).The reagents were given time to dissolve and mix. The polymer was continuously removed by winding of the glass rod until no further polymer was formed. The second solution B was prepared by adding 10 cm³ of sebacoyl chloride in cyclohexane in a beaker and adding 100 mL of hexamethylenediamine in warm water to melt it (225°C). The polymer was continuously removed by winding of the glass rod until no further polymer was formed. The third solution C was made by mixing equal amounts of 5 cm³ each of adipoyl chloride and adipoyl chloride sebacoyl chloride in in cyclohexane in a beaker and adding 100 mL of hexamethylenediamine in warm water to melt it (215°C).
As described by (David and Antonios, 1999), all the precautions were carried to ensure that there was accuracy and no hazards from the beginning to the end. All the measurements were taken care of to ensure no irregularities were encountered during the experiment.
The set experiment was conducted as shown in figure 1, 2, 3, and 4. The figures indicate the procedures that were followed for the experiment.




Results
The calculation of the reagents is as shown:
- 10 mL of adiploy x 183. 03 g = 2.9
- 100 ml of adipoyl x 5 mL x 1.25
- 10 mL of sebacoyl x 239.14g = 4.1
- 100 ml of sebacoyl x 5 mL x 1.14
- The third solution will therefore be equal to 3.5 moles.
By using the Beilstein test for halogens, it was clear that the flame did not turn into green. This was a clear indication that there are no halogens. These was done by heating a copper wire and touching the sample and returning the wire to the Bunsen flame. The soda lime test for nitrogen was done by placing a small piece of sample in to the bottom of a small test tube and covering it 1 – 2 cm3 of soda lime. Then the base of test – tube was strongly heat in the flame. Since there was no presence of ammonia, there was no change in the litmus paper for the three samples from the experiment. It was also clear for the three samples that, none of the polymers could float on water even when very wet. When small piece samples were heated using a Bunsen burner flame, none of them could ignite and no smoke was registered from any sample. The results show that Nylon 6 – 6 is formed from the first solution. This is because the length of the carbon chains that are formed by adipoyl chloride. The second solution B gives a diamine and this is present in excess and which react with the hydrogen chloride that is eliminated.
The chain of reaction is as shown below.
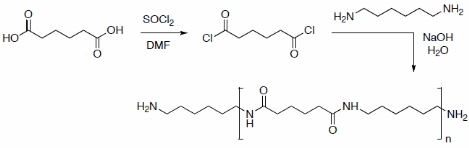
The equation for this reaction is:
H O O
│
║ ║
H2N-CH2-CH2-CH2-CH2-CH2-CH2-NH + HOC-CH2-CH2-CH2-CH2-COH
H O O
│
│ ║ ║
H2N-CH2-CH2-CH2-CH2-CH2-CH2-N-C-CH2-CH2-CH2-CH2-C-OH + H2O (Nylon 6,6)
This can also be represented as:
H H O O H H O O H H O O
│ │ ║ ║ │ │ ║ ║ │ │ ║ ║
n -N-(CH2)6-N-C-(CH2)4-C-N-(CH2)6-N-C-(CH2)4-C-N-(CH2)6-N-C-(CH2)4-C- + 5n H2O
The experiment can be summarized in a table form as below
Discussion
The properties of polyamides and the polyester developed shows that they both have inter molecular forces that are developed by hydrogen bonding. This is shown in a diagram 5 below. The hydrogen bonds are said to interact well with the oxygen molecules forming a chain of carbonyl oxygen of nylon chain.
Acid chlorides are said to be more reactive than chlorides and this makes allows polymerization to form Nylon 6, 10.
Conclusion
The polymers that were formed shows that fibers are very good and outstanding in durability and have very good physical properties. The fact that no ammonia gas is produces show that their applicability in textile industry is warmly welcome. The other properties of resistance to shrinking and stretching make it more ideal in cold countries and the less labour involved in the pressing of clothes. Across the globe, the temperatures vary and the properties of the nylon help greatly in textile industry. Since nylon has uniform and scuff abilities that exhibit all chemical resistance. This enables the nylons to remain the strongest synthetic fiber which is common in use in most parts of the globe. As reported by (Guoping, et al, (2001), polymers have good mechanical and thermal properties that are very crucial in thermoplastic engineering and automobile companies.
List of References
David J. M. and Antonios G.M., (1999) Growing New Organs April issue. Special issue, Columbia, San Antonio Express, pp. 42 – 44.
Guoping C., Takashi U., Tetsuya T., (2001) Development of Biodegradable porous scaffolds for tissue engineering. Web.
Ravez A. J., (1967) Organic Chemistry of Macromolecules, Marcel Dekker: New York, Chapter 15, p.43 – 47.
Shakhashiri B. Z., (1983) Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, Vol.1, p.213-215.
Shoufeng Y., Kah-fai L., ZhaoHui D., and Cheekai C., (2001) The Design of Scaffolds for use in tissue Engineering Part I. Traditional Factors. Tissue Engineering. Volume 7, Number 6, pp. 17 – 20.
Question
- The longitudinal modulus of apodemes hind legs with a 25 % volume and 80 GPa and a matrix of 120 MPa is:
ECL = EFVF + EMVM Therefore, Ecl = 80 (1- 0.25) + 1.2 (0.25) = 40.3 GPa
- Transverse modulus of the apodemes is:
1 = Vm + Vf
Ecl Em Ef
Ecl = (1.2 x 80) / ((0.25)80 + (0.25)1.2) = 96 / 20 + 0.3 = 4.7 GPa
- The assumption that is made during the calculation is that all pproperties under the same strain are very anisotropic. This makes them vary significantly just like woods. However, the muscular strain of the apodemes is almost similar in all cells. The amount of stress applied on the apodemes is relatively proportional to the yields strain in the same direction. Moreover, the cell walls bend and their properties are proportional to (density) 2.
- The chitin skeleton is arranged in a form of synthesised proteins to make fibres. The fibres are closely tailored by carefully ordering amino acids and controlled by inherent properties of material. The skeleton use a lot of energy and the position of fibres are optimized arranged within the protein matrix.
- These properties make the skeleton firm execarcebate the toughness since the structure is formulated in a way that is relevant to any kind of strain. The structure thus reveals that there are no weak interfaces since each line is carried by different forces.
- The amount of compressive stress is calculated by Force x cross sectional diameter / length of the compressed stress
Therefore, the stress
(50 × 5 × 100) / (10 × 100 × 10) = 2.5 n/m2
- The elastic energy stored is calculated as,
(50(3) + 0.05(10)) x 100 = 1505 N
- The stored energy is used to operate the Inner hinge ligament of bivalves and offers a passive antagonist to shell adductor muscles
- It is safe to store this energy here because the bivalves has amino acid composition that contain large number of helix breakers and there is intense random coil conformation 3a.
The young modulus of the flamingo is at 3.
- He calculations will be (30 × 10 × 100) / (25 × 10) = 120
- the longitudinal moduli of the feature will be at 50 x 0.35 + 0.35 x 0.65 = 17.5 + 0.3 =17.8
The transverse moduli will be 3 x 0.35 + 0.65 x 0.35 = 10.8
- the stress in the femur is (80 × 25) / (45 × 10) = 4
The strain is (80 × 45) / 25 = 144
- the energy stored in the femur is (800 × 45 × 10) / 25 = 1440 newtons.