The aim of this experiment is to show how natural enzymatic reducing agents are as effective as the chemical ones.
A reduction process is characteristically the gain of two hydrogen atoms or the loss of an oxygen atom, or both (Fox & Whitesell, 2007). This results to a structural configuration conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. Curvularia Lunata conceived the Prelog’s rule in the reducing of decalones where the hydrogen transfer to the S alcohol will continue on the re-face of the prochiral ketone, in this case the reduction process gives the S enantiomer (Chenevert & Soniat, 1985 & Zhu, et al.2006).
Chenevert & Soniat, (1985) confirm that it is possible to determine the absolute structural make-up of the consequent product after microbial reduction on a carbonyl group that has the large group L and a small group S to the alcohol through use of Prelog’s rule (1599). Prelog’s rule (1599) states “In the presence of tertiary aliphatic amine, reaction of phenylmagnesium bromide with ethyl (S)-(+)-3-hydroxybutanoate the Grignard reaction with an ester gives a ketone as the major product hence the yeast reduction of 1-phenyl-1, 3-butanedione gives the title compound with S configuration”.
Since it has been established that yeast has three of carbonyl-reducing enzymes and with the various abilities of enantio to differentiate, it is advisable that care should be taken when conducting the process. During this series of reactions, the reduction process has the potential to convert achiral compounds to chiral ones (Fox & Whitesell, 2007). The analysis of nuclear magnetic resonance is able to establish the result due to composition of enantio. Chenevert & Soniat, (1985) gives an example of how nuclear magnetic analysis can be applied using tris [3-(heptafluoropropyIhydroXymethylene)-d-camphorato] europium (III) as a chiral shift reagent. According to Ohta et al. (1990) “The nuclear magnetic resonance analysis usually needs the use of racemic 3-hydroxy- 1 -phenyl- 1-butanone, which can be prepared by condensation between acetaldehyde and benzoylacetic acid”.
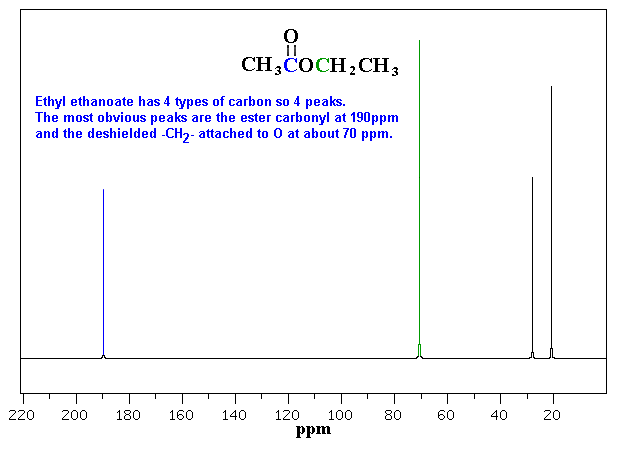
In the results captured, Ethyl ethanoate has four types of peaks recorded, in this experiment these are at 3.042; 2.798; 1.818 and 2.000, sequentially.
3453 0.022; 2976.334 240.638; 1716 0.1113; 1448.296 0.000; 1407.458 0.000; 1372.144 183.660; 1248.3171 81.456; 1296.906 260.605; 1177.895 709.159; 1027.599 453.953; 947.685 147.942; 844.333 52.179; 809.806 47.158; 725.111 0.000
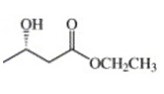
Asymmetric synthesis has relied on the use, as starting materials, of optically active compounds that are members of the so-called “chiral carbon pool,” i.e. easily available chiral substances produced by living organisms including amino acids, terpenes, carbohydrates, etc (Chenevert & Soniat,1985 and Sih & Chen,1984). According to Seebach et al.( 2006), the spectral properties of (S)-( + )-ethyl 3-hydroxybutanoate are as follows: IR2a (film) cm−1: 3440, 2980, 1730, 1375, 1300, 1180, 1030; 1H NMR2b (CCl4) δ: 1.15 (d, 3 H, J = 6.5, CH3), 1.28 (t, 3 H, J = 7 Hz, CH3), 2.35 (d, 2 H, J = 6.5, CH2CO), 3.15 (s, 1 H, OH), 4.05 (q, 2 H, J = 7, CH2O), 4.15 (m, 1 H, CHOH). The reduction process gives a (S)-(+)-3-hydroxy-1-phenyl-1-, which is 33 percent pure, product which entire depends on the starting materials.
Kuramoto et al. (1999) attests a similar case. The specific Enantiometric purity of product can be calculated given that the enantiometrically pure (S)-ethyl 3-hydroxy-butanoate has (α)25D=+43.5(c=1,CHCl3) and that [α]D=αIXC where α]D is specific rotation; α is the observed rotation in degrees; I is the path length in dm and c is the concentration in g/ml. This gives a product of 0.3234.
In conclusion, Baker’s yeast is a chiral reagent that will catalyze and cause chirality to a molecule. Reaction changes that are bio-catalyzed are useful since are acclimatized to the surrounding conditions of temperature and pressure.
References
Banora, G.M., Drily, S., Format, C., Nitti, P., & Pittance, G., 2005. Baker’s Yeast Reduction of PEG-Linked Acetoacetate. Letters in Organic Chemistry. Web.
Chenevert, R. & Hiboutoct, S., 1986. Enantiospecific synthesis of optically pure (S)-(+)-3-hydroxy-1-phenyl-1-butanone, by bakers’ yeast reduction. Web.
Fox & Whitesell. Experiment 7 – 2007: Reduction of Ethyl Acetoacetate: Achiral and Chiral Reduction. Web.
Hamdania, M., De Jeso, B., Deleuzea, H., Sauxa, A. & Maillard, B., 1993. The product of Baker’s yeast reduction of ethyl 2-chloro-3-oxobutanoate as a precursor of the 1-ethoxycarbonyl 2(S)-hydroxypropyl radical. Web.
Kuramoto, T., Iwamoto, K., Izumi, M., Kirihata, M., & Yoshizako, F., 1999. Asymmetric Reduction of Ethyl 2-methyl 3-oxobutanoate by Chlorella. Japan Society for Bioscience, Biotechnology, and Agrochemistry. 63(3), p.598-601. Web.
Ohta, H., kobayashi, N. & Sugai, T., 1990. Reduction of Acyl Enolates of α-Substituted β-Keto Esters by Bakers’ Yeast. Japan Society for Bioscience, Biotechnology, and Agrochemistry. 54 (2), p. 489-4923. Web.
Seebach, D., Sutter, M. A., Weber, R. H. & Züger, M. F., 2007. Yeast Reduction of Ethyl Acetoacetate: (S)-(+)-Ethyl 3-Hydroxybutanoate [Butanoic acid, 3-hydroxy-, ethyl ester, (S)]. Web.
Salvi, N. A. and Chattopadhyay, S. (2004). Rhizopus arrhizus mediated asymmetric reduction of alkyl 3-oxobutanoates. Elsevier Ltd. 15(12), p. 3397-3400. Web.
Sih, J. and Chen, C-S. (1984). Microbial Asymmetric Catalysis – Enantioselective Reduction of Ketones [New Synthetic Methods (45)] Web.
Spectroscopy, n.d.. Interpreting C-NMR Spectra. Web.
Zhu, D. Yang, Y. and Hua, L. (2006) Stereoselective Enzymatic Synthesis of Chiral Alcohols with the Use of a Carbonyl Reductase from Candida magnoliae with Anti-Prelog Enantioselectivity. American Chemical Society. 71 (11), p 4202–4205. Web.
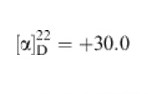 (c0.77, CHCl3)
(c0.77, CHCl3)