Introduction
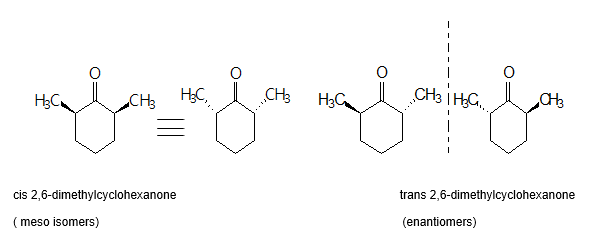
The aim of this experiment was to prepare a stereoisomeric mixture of secondary alcohols from the reaction of cis and trans 2, 6-dimethylcyclohexanone with sodium borohydride. 2, 6-dimethylcyclohexanone is a mixture of cis and trans isomers each containing a ketone functional group. The cis:trans ratio of 2,6-dimethylcyclohexanone used in this experiment was 80:20(cis:trans). The trans isomer has two distinct enantiomers while the cis isomer is a meso compound as shown in the diagrams below:
Nucleophilic attack of the H– ion(from NaBH4) on the electrophilic carbon atom of 2, 6-dimethylcyclohexanone can occur either from front face or from the back face. This result to the reduction of the ketone carbonyl group (sp2) to a secondary alcohol in which the carbonyl carbon is sp3 hybridized (Hathaway 1623). The reaction mechanism is as shown in the diagram below.
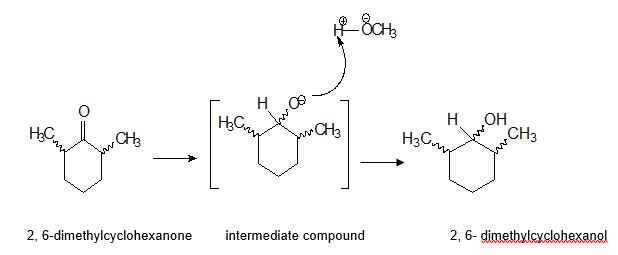
The products of this nucleophilic acyl addition reaction are 2, 6-dimethylcyclohexanol isomers. Each product contains two chiral centers at C2 and C6 and as shown by the spiral lines draw in the structures above. Depending on the configuration of the atoms/group of atoms attached to chiral center, we get either R- or S-diastereomer.
Since diastereomers have distinct free energies associated with them, they can be separated and characterized experimentally by chromatographic techniques i.e. gas chromatography and spectroscopic methods i.e. IR, NMR. On the other hand, enantiomers have identical free energies and cannot be separated by ordinary means (Hathaway 1624).
The products were analyzed using IR spectroscopy method to determine the functional group transformation that occurred (from ketone to alcohol). Gas chromatography analysis was done to examine the stereochemical distribution of the products i.e. cis versus trans resulting from the reaction.
Procedure
Synthesis of 2, 6-dimethylcyclohexanol
10ml of methanol and 0.5g of 2, 6-dimethylcyclohexanone (80:20 cis: trans mixture) were added to 50ml Erlenmeyer flask containing a magnetic stirrer bar. The stirrer was placed in the hood with two ring stands positioned on either side. An ice bath (~½ – ¾ full) was placed on the stirrer and the flasks were inserted in it and clamped on the ring stands. The stirrer/hot plate was then turned on (not heat).
100mg of sodium borohydride was added in 2,50mg portions to the flasks as the stirring continued. After adding all the NaBH4, the reaction was allowed to subside for approximately 5-10 minutes before removing the flasks from the ice bath. The mixture was then stirred for another 15minutes at room temperature. 5ml of 10% NaOH, 5ml of de-ionized water and 15ml of hexane were then added to the flask. The contents were stirred for 3-5 minutes. The stirrer was then removed and the contents were transferred into a 125ml separation funnel clamped on a stand. The organic layer containing the products was separated from the aqueous layer in clearly labeled 50ml flasks.
The aqueous layer was returned into the separatory funnel and 10ml of hexane was added. The funnel was stoppered and the contents were shaken for 1-2 minutes and then allowed to stand. The organic layer was dispensed into flask labeled “organic layer.” This washing was repeated once more and the remaining aqueous layer was drained into the flask labeled “water layer.” 100mg of magnesium sulfate (drying agent) was then added to the beaker labeled “organic layer” and the contents swirled. The mixture was then filtered into a pre-weighed, clean, dry 125ml Erlenmeyer flask, separating the drying agent from the organic layer.
A water bath (~½ – ¾) full of tap water was then placed on the stirrer/ hot plate. The flask containing the organic layer was clamped in to the water bath. The hot plate was switched on and the contents heated gently until all the hexane evaporated, leaving a clear to slightly yellow oil as the product. The flask was then removed from the water bath and wiped to dry. It was then weighed to determine the mass of the product and therefore the percent yield.
Analysis of 2, 6-dimethylcylohexanol
An infrared (IR) spectrum of both the product and 2, 6-dimethylcyclohexanone was run to determine the functional group transformation. Further, a gas chromatography analysis was done determine the products of the reaction as well as their stability.
Test of alcohols (Jones Oxidation)
Four clean and dry test tubes (75mm X 12mm) were set on the test tube rack and labeled # 1-4. In test tube #1,2 drops of cyclohexanol (+control) were added, test tube #2,2 drops cyclohexanone(-control) were added, test tube #3,approximately 10mg of 2, 6-dimethylcyclohexanone were added and in test tube #4,10mg of the product were added. 1ml of acetone was added to each test tube and the mixture swirled to dissolve the compounds. One drop of the Jones reagent was then added to each test tube and the color change observed after about 1 minute.
Bradys test for ketones
Eight clean and dry test tubes (75mm X 12mm) were placed in test tube rack in the hood. The test tubes were labeled #1-8 and reagents were added as follows:
In test tube #1, 2 drops of cyclohexanone (+ control), test tube #2, two drops of cyclohexanol (- control), test tube # 3,10mg of 2, 6-dimethylcyclohexanone and in test tube #4,10mg of the product was added. 2ml of ethanol were added to each test tube and the contents swirled to dissolve the compounds. 2ml of the Bradys reagent were added to test tubes #5-8. The contents of tube #1were added to tube #5, tube #2 to tube #6, tube #3 to tube #7 and tube #4 to tube #8 respectively. The contents were swirled to mix and the color of the precipitate noted.
Results/observations
Mass of the product=0.34g
Table 1; Test for alcohol (Jones oxidation)
Table 2; Bradys test for ketones.
Table 3; Major IR spectrum peaks for 2, 6-dimethylhexanone
Table 4; Major IR spectrum peaks for 2, 6-dimethylhexanol
Discussion
Color changed from orange to blue in both test tube #1 and #4 confirming the presence of alcohol functional groups (positive test to Jones oxidation test). The color change in test tube #4 confirmed that the ketone functional group in 2, 6-dimethylhexanone was transformed to an alcohol in course of the reaction. Test tube #2 and #3 tested negative because they contained ketones. In the Bradys test for ketones, a yellow precipitate was observed in both test tube #5 and #7 indicating the presence of ketone functional groups. A negative test in test tube #8 confirmed that the transformation of the ketone to an alcohol.
The IR spectra of 2, 6-dimethylhexanone shows strong absorption peak between 1710-1720 cm-1due to C=O stretching characteristic for ketones. The weak peaks around 1350-1360 cm-1are due to strong CH3 bending and the C-C-C bending give rise to the peaks at 1100 cm-1 region.
The IR spectra for 2, 6-dimethylcyclohexanol shows a strong broad peak at around 3450 cm-1due to stretching of hydrogen bonded O-H bond characteristic of alcohol functional group. In the region between 980-1200 cm-1 sharp peaks resulting from C-O stretching was observed. Other medium peaks in 1330-1430 cm-1region result from O-H bending (in-plane bending) in 2, 6-dimethylcyclohexanol. The strong peak at around 2800-2975 cm-1 results from stretching of C-H bonds.
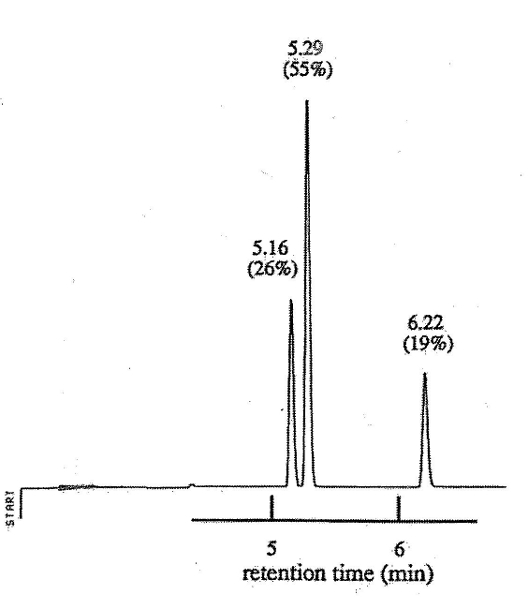
From the calculated values of the steric strain energy, D and E (0 Kcal) are the most stable conformers while F and H (2.4Kcal) are the least stable. The highest peak on the chromatogram corresponds to the most stable conformers.
Conclusion
The Jones test, Brady’s test and the IR spectrum confirm that 2, 6-dimethylcyclohexanone was reduced to 2, 6-dimethylcyclohexanol. A yield of 66.93% of the product was obtained showing that the method was effective. The IR spectrum of the product shows strong peaks resulting from OH functional groups illustrating functional group transformation. Conformational analysis shows that the cis isomer of 2, 6-dimethylcyclohexanol (D and E) are the most stable (0Kcal steric strain energy) while F the least stable (2.4Kcal steric strain energy). Conformer has a distribution of 54.17% and corresponds to the
Work Cited
Hathaway, Bruce. Reduction of 2, 6-dimethlcyclohexanone with sodium borohydride revisited. Journal of Chemistry Education 75.12 (1998): 1623-1624.