Purpose: The primary purpose of the given experiment is to observe how specific the effects of increasing enzyme concentration, pH, and temperature are when utilizing potato juice and hydrogen peroxide.
Procedure
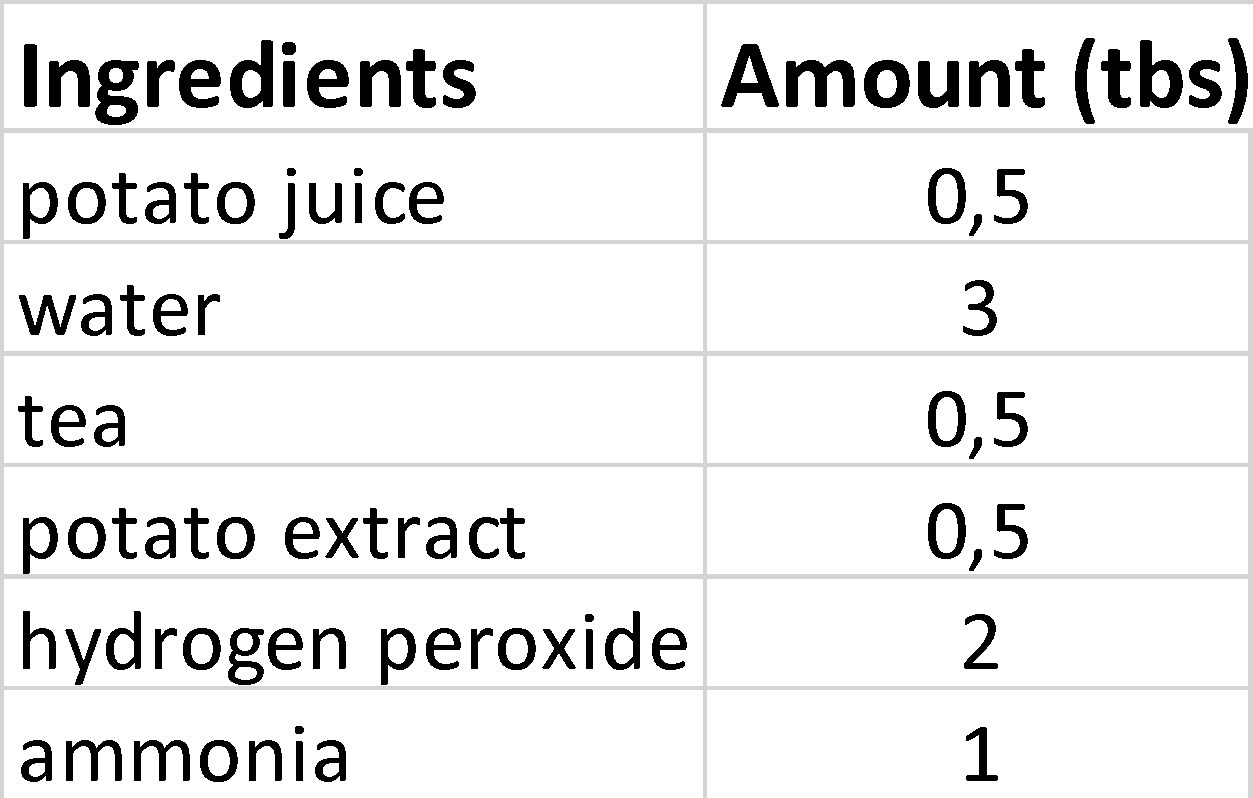
The list of ingredients with their corresponding amounts can be observed in Table 1 below. It is important to note that tea and potato extract elements were the primary enzyme substrates for the given experimental analysis on specificity. The ammonia and lemon juice levels were my independent variables in the Enzymes Denaturation by pH experiment. The bubble reaction generated by ammonia was considered a dependent variable. The key observation is that no response occurred with lemon juice. One tablespoon or tbs ammonia and lemon juice served as the core control variables. The temperature change for the potato juice, hydrogen peroxide, and water was my independent variable for the enzyme denaturation by temperature experiment. The bubble reaction induced by the low-temperature chemicals was the basic dependent variable however hot water mixed with ambient temperature did not cause a chain reaction. The amount of cold/warm potato juice, water, and hydrogen peroxide I put into the cup served as my control variables.
The potato extract and the brand of tea I used in the enzyme-substrate specificity assay. The bubble response at the top of the cup created by the potato juice, water, and tea was my dependent variable. The period of time needed to whisk the potato juice, water, and tea was my control variable. The reduction in water in the cup was my independent variable for the enzyme concentration experiment. The rise in bubbles caused by adding more potato juice and less water was my dependent variable. Adding less water and more potato juice, as well as the amount of hydrogen peroxide, was my control variable.
Hypothesis
The height of the bubbles created by potato juice, tea, and water will be lower than the bubble response induced by hydrogen peroxide. More potato juice and less water in the cup would raise the bubble height. The height of the bubbles created by one tablespoon of ammonia would be higher than that of the lemon juice vial. The bubble height with cold chemicals will be higher than that with room temperature chemicals.
Results
When potato juice is mixed with hydrogen peroxide, the bubbles rise to the top of the pill vial. The height of the bubbles may be increased by adding more potato juice and decreasing the amount of water (Bisswanger, 2017). The bubble reaction will be stopped if the temperature changes from cold to hot.
Reference
Bisswanger, H. (2017). Enzyme kinetics: Principles and methods. Wiley.