What is a Double-Blind Test?
Blind tests are a helpful tool in research practice. A double-blind test is a randomized trial in which neither the researchers nor the subjects are fully informed about who is receiving the intervention and which intervention (Practical Psychology, 2022). This type of study increases the results’ objectivity, validity, and a more detailed view of the parameter under investigation (Ranganathan & Aggarwal, 2018; Probst et al., 2019).
Applications of Double-Blind Tests: Where Can We Use Them?
Double-blind tests are used when data on control and study group membership are not expected. This type of study is used in medicine and psychology because it allows for the control of placebo effects. In this case, the placebo effect can play an additional role in increasing the effectiveness of the intervention in the study group (Simple Learning Pro, 2015).
Graphics and Data Examples of Double-Blind Tests
Figure 1 provides a participant selection scheme for a blinded study demonstrating the concept of the study.
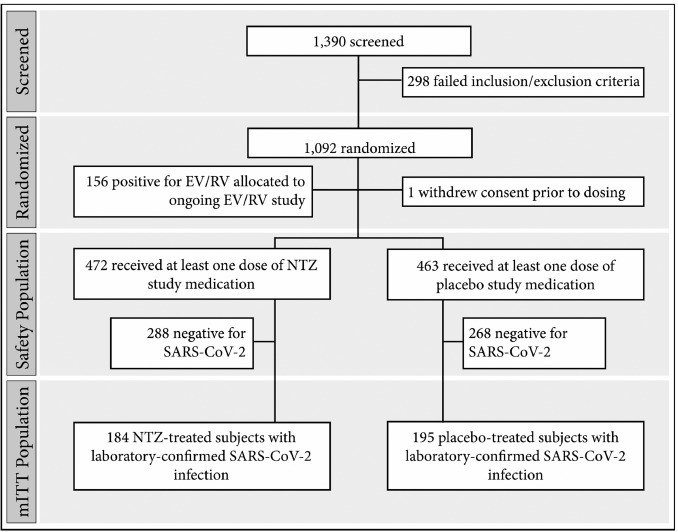
The diagram shows that in blind studies, the selection of participants is as rigorous as in open studies but becomes more closed as one moves toward the moment of intervention. Participants are prepared before the intervention, making them blind to increase validity. Similarly, in Figure 2, one can see how the data are entered into the table to process the results of the blind studies.
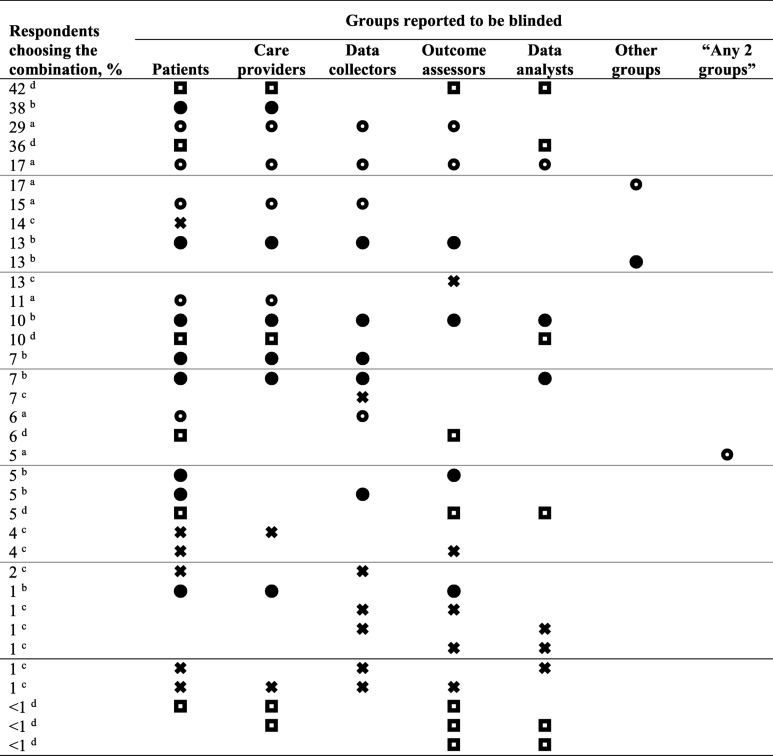
What Are the Benefits of a Double-Blind Test?
It is established that in blind studies, the level of results obtained and their quality will be higher. Probst et al. (2019) point out that the blind approach is essential for shaping and anchoring evidence-based findings, which are then integrated into medical theory and practice. Penic et al. (2020) investigated the fact that blinded randomized trials provide a high degree of engagement and the ability for scientists to remain unbiased. Consequently, the benefits of double-blind studies are clear, and their use should be more widespread.
References
Lang, T. A., & Stroup, D. F. (2020). Who knew? The misleading specificity of “double-blind” and what to do about it. Trials, 21(1), 697. Web.
Penić, A., Begić, D., Balajić, K., Kowalski, M., Marušić, A., & Puljak, L. (2020). Definitions of blinding in randomised controlled trials of interventions published in high-impact anaesthesiology journals: A methodological study and survey of authors. BMJ open, 10(4). Web.
Practical Psychology. (2022). What is a double blind study? (definition + examples) [Video]. YouTube. Web.
Probst, P., Zaschke, S., Heger, P., Harnoss, J. C., Hüttner, F. J., Mihaljevic, A. L., Knebel, P., & Diener, M. K. (2019). Evidence-based recommendations for blinding in surgical trials. Langenbeck’s archives of surgery, 404(3), 273–284. Web.
Ranganathan, P., & Aggarwal, R. (2018). Study designs: Part 1 – An overview and classification. Perspectives in clinical research, 9(4), 184–186. Web.
Rossignol, J. F., Bardin, M. C., Fulgencio, J., Mogelnicki, D., & Bréchot, C. (2022). A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine, 45. Web.
Simple Learning Pro. (2015). Placebo effect, control groups, and the double blind experiment (3.2) [Video]. YouTube. Web.