Introduction
At the beginning of October, a proposal to organize an effort within the Laboratory Corporation of America Holdings to repeal or amend the upcoming changes in Medicare clinical laboratory fee schedule rates was created. The proposal was approved by the Chairman and Chief Executive Officer David P. King shortly after it was presented to him. No additional funding for the project was required because all of the required actions could be completed in a short amount of time and utilizing the already existing resources.
However, the timetable for the project is also short and requires the participation of all the members of LabCorp, and especially medical professionals. Currently, the project is going according to the plan under the helm of David King. An early issue occurred after the initial proposal was approved but was soon resolved with no major effects.
Participating in the Effort
The effort consists of three major elements. The first is focused on informing the staff about the issue at hand through the information channels within the company. E-mails were sent to all the employees of LabCorp with the overview of the issue and what they can do to prevent it from having a negative effect on them and their patients. The issue of new Medicare Clinical Laboratory Fee Schedule is likely to have a severe effect on the availability of lab tests to clinics and could result in large-scale layoffs to make the service more sustainable through downsizing.
The second step involves the participation of the employees in the effort to repeal or amend the new rates. To ensure that the information is correctly presented and delivered to its destination, an online service provided by the American Clinical Laboratory Association is recommended to all the employees of the company. Their goal is to fill in their personal information into the form provided by ACLA on its website, after which, it would be applied to special form letters and sent to the relevant state representatives that could possibly repeal the new rates or at the very least amend them based on factual information, rather than an unreliable research paper. The process of filling out the form should not take more than 15 minutes, after which the employees would be free to continue their work day.
The third step concerns the actions of the company’s legislative affairs person Samuel Eberts III. He will be tasked with contacting the Washington representatives on the issue to inform them further and evaluate the effect that the effort has on the problem.
Benefit to the Employees and the Corporation
The initial attention to the issue of the new Clinical Laboratory Fee Schedule rates was brought by the American Clinical Laboratory Association on September 22, 2017. The association released a statement on the proposed PAMA Medicare Payment Rates for Clinical Laboratory Tests. The authors of the press release expressed a deep concern for the lack of accuracy in draft rates that were published under the PAMA.
The review of the draft rates revealed that they were not based on the realities of the market and would inflict severe cuts that were beyond the plan proposed by Congress. While no concrete data was presented, the extremely selective nature of the research paper suggests that the cuts proposed by it would only serve to inflict harm on laboratories across the nation (“ACLA Expresses Serious Concerns”).
Subsequently, on September 25, 2017, the chairman and CEO of LabCorp expressed his support of ACLA and presented additional data from the review of the draft rates. The issue of the rates was caused by the faulty process that Centers for Medicare and Medicaid Services followed during their research of the market. In fact, only 1% of all laboratories in the United States were chosen to report the data, with the majority being independent labs.
He estimated that the impact of the new draft rates could negatively affect Medicare beneficiaries, reduce the availability of lab tests, and prevent innovation in research and development (“LabCorp Objects to Proposed PAMA”). The proposed rates vary in the payment changes with some procedures becoming 751% more expensive, while others are reducing by 95% (Fig 1). This data is based on a very limited sample, and therefore the proposed changes would not reflect the way that the majority of laboratories operate.
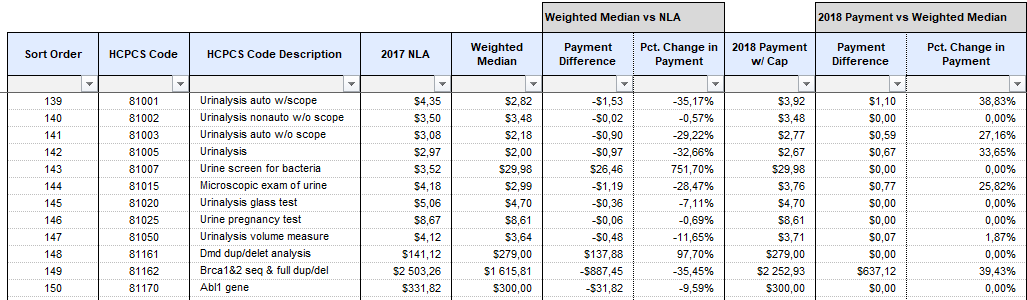
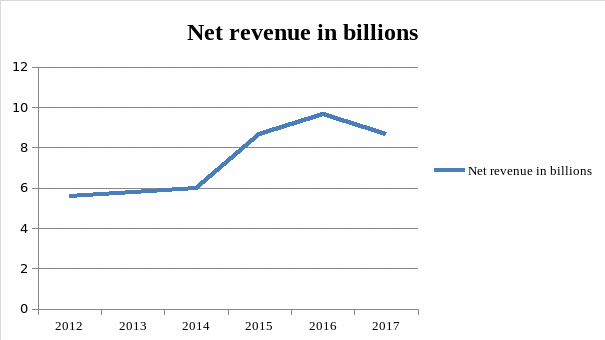
The proposed effort was designed to support the actions of ACLA in opposition to new rates which are scheduled to become active on January 1, 2018. While the David King stated that the company is prepared for the case in which these rates are implemented in their current form, around 10% of all LabCorp revenue is based on Medicare, with $9,5 billion in net revenue that the company recorded in 2016, it would lead to a decrease in almost a billion dollars of revenue for the company (Fig 2). If this effort succeeds, the company will benefit by continuing its current revenue flow, while the employees would not have to be laid off to make up for the loss and patients would have access to laboratory tests in a greater number of cases.
Problem Encountered During Implementation
During the first step of the implementation, an issue with the information system arose unexpectedly. While every employee of the company was used to receiving important information by E-mail, the system was not designed with a focus on the prolonged interaction of tens of thousands of employees on a single issue. This led to a crash of the internal E-mail server which could affect the timetable of the effort. However, a backup server was utilized to handle the load, and the issue was resolved without a significant loss of time. Alternatively, a secondary server could be purchased, but with the limited timetable of the task, it would be a costly and inefficient solution to the problem.
Timetable
- October 10: All of the employees will be sent information about the problem, including links to the new rates and how they are likely to affect the company, and the industry as a whole. Underneath the information, a link to the ACLA information submission form will be present with instructions on how to fill it out.
- October 10 – November 25: All of the U.S. employees of LabCorp will fill out the form which will be sent to the relevant state representatives in their area. While the new rates are not set to be implemented until 2018, it is essential that the form letters are sent out as soon as possible.
- November 25 – January 30: The legislative affairs person of the company will contact the representatives in Washington on a weekly basis to monitor the process and evaluate the effect of the effort. The success will be measured by the way in which the new rates are changed before and after their possible implementation.
Conclusion
The effort is currently proceeding on schedule. I am glad that the project was approved in such a timely manner and hope that the effort will prove successful. The possible loss of a $1 billion makes this a serious issue.
Works Cited
“ACLA Expresses Serious Concerns.” ACLA, 2017. Web.
“LabCorp Objects to Proposed PAMA.” BusinessWire, 2017. Web.
“LabCorp on the Forbes Growth Champions List.” Forbes, 2017. Web.
“PAMA-Regulations.” CMS. 2017. Web.