Welcome to our essay sample on synthesis of triphenylmethanol from the reaction of the Grignard reagent! Here, you’ll find information on Grignard reaction procedure, TLC analysis, and other aspects of the experiment.
Abstract
In this experiment, triphenylmethanol was synthesized from the reaction of the Grignard reagent (phenyl magnesium bromide) with benzophenone (a ketone) and hydrolysis with HCL. The final product was purified and characterized using TLC and IR spectroscopy. The final product was weighed and the percentage yield calculated. All the apparatus for this experiment were kept completely dry as protons easily hydrolyze the Grignard reagent to a hydrocarbon. The melting point of triphenylmethanol was determined and checked against the recorded value to determine the purity of the product. The impurities generated in this reaction include biphenyl (from the reaction of phenyl radicals) and benzene from the protonation of the carbonation.
Introduction
Grignard reaction allows the formation of carbon-carbon bonds using organometallic intermediate (Grignard reagents). Grignard reagents are prepared by reacting magnesium metal with alkyl or aryl halides in aprotic solvents such as diethyl ether or tetrahydrofuran. They have a carbon-metal bond, which changes the polarity of a carbon atom from the partial positive charge (in aryl and alkyl halides) to the partial negative charge in Grignard reagents. They are strong bases as well as strong nucleophiles and readily react with electrophilic species. In the presence of acidic protons, water, or alcohol the Grignard reagent breaks its organometallic bond leading to the formation of a hydrocarbon. It is therefore important to maintain very dry conditions in reactions where the Grignard reagents are involved.
Electrophilic functional groups such as carbonyl-containing compounds, alkyl halides, and alcohols readily react with the Grignard reagent through nucleophilic acyl addition and nucleophilic acyl substitution mechanism. The reaction mechanism for the reaction between alkyl halides and Grignard reagent is similar to that involving a proton and the Grignard reagent. A new bond forms between the nucleophilic carbon of the Grignard reagent and electrophilic carbon of the alkyl halide. Aldehydes and ketones react strongly with the Grignard reagent via nucleophilic acyl addition mechanism forming alcohol as the product. The reaction of the Grignard reagent with different species is given in the figure below.
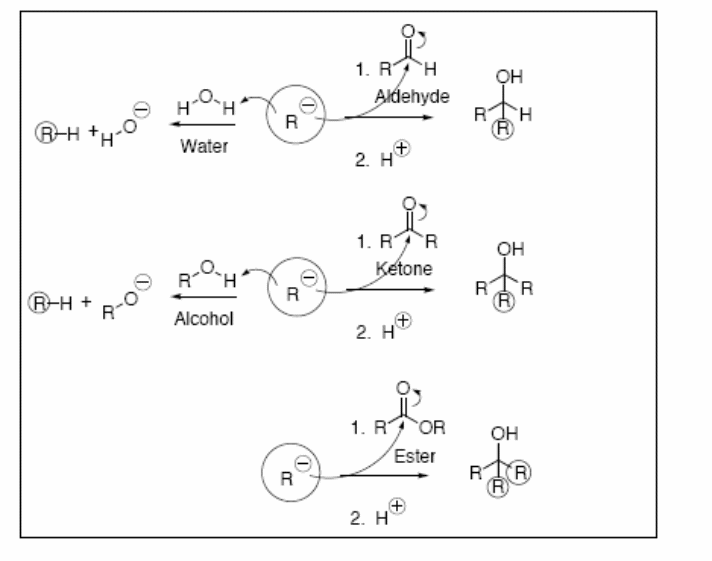
Nucleophilic acyl substitution reaction occurs when the starting material is an ester, amide, or acid anhydride. In this case, the electrons on the negatively charged oxygen atom are used to reform the carbonyl pi bond, thereby forcing the C-N or C-O bond of the ester, amide, or anhydride to detach as a leaving group generating a ketone intermediate. The ketone intermediate reacts with more Grignard reagent leading to the formation of a highly substituted alcohol.
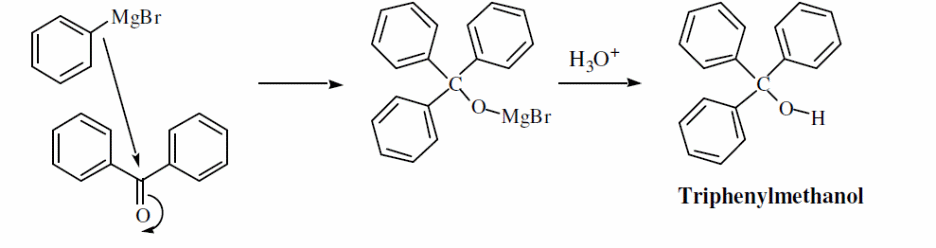
Triphenylmethanol is prepared from benzophenone using a Grignard reagent e.g. phenyl magnesium bromide through nucleophilic acyl addition mechanism. “Magnesium alkoxide is formed as an intermediate, which is then hydrolyzed by HCL to give an ether soluble triphenylmethanol and a water-soluble salt of MgBrCl” (Addison 446). This occurs as shown by the reaction scheme below.
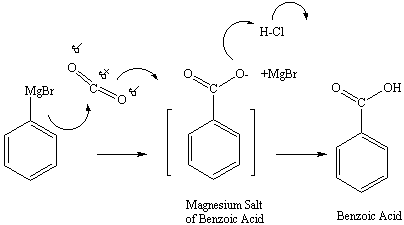
Carbon dioxide reacts with the Grignard reagent especially. A 1:1 ratio of carbon dioxide with phenyl magnesium bromide yields an MgBr salt of benzoic acid. The reaction of the salt with HCL yields benzoic acid and the water-soluble MgBrCl salt as shown in the figure below:
Below is the procedure for the preparation and characterization of triphenylmethanol starting with the preparation of the Grignard reagent (phenyl magnesium bromide) and subsequent reaction with benzophenone.
Procedure
Preparation of the Grignard Reagent
All the glass apparatus were first dried in the oven for about 10minutes.
Approximately 0.5g of magnesium turnings and a magnetic stir bar were put into a dry 100ml round-bottomed flask and then placed in the drying oven for 30minutes. The flask was then removed from the oven, clamped on to a ring stand, and fitted on the heating mantle. A reflux condenser was then inserted and the drying tube was immediately fixed to the top of the reflux condenser.
The flask was placed on the stirrer/hotplate and the contents allowed to attain the room temperature before proceeding. 3.5g of bromobenzene was added to a dry 50ml Erlenmeyer flask and dissolved in 5.0ml of anhydrous diethyl ether. A glass powder funnel was then used to transfer bromobenzene and ether to the Erlenmeyer flask where the contents were swirled to dissolve bromobenzene. Bromobenzene solution was transferred to the 100ml round-bottomed flask when it cooled using a funnel. A cloudy solution formed indicating that the reaction had begun.
An additional 10ml of ether was added to the flask using a glass funnel. Water was then allowed to flow through the condenser. The reaction was allowed to continue steadily for 15 minutes until all the magnesium dissolved and the contents turned brown/cloudy. The level of ether was kept constant in the flask by adding more as it evaporated. The condenser was replaced with the drying tube and the contents were kept aside.
Preparation of Triphenylmethanol
3.7g of benzophenone were placed into a clean dry 150ml beaker followed by 10ml of ether. The beaker was swirled gently until all the benzophenone dissolved. The condenser tube and the drying tube were removed from the reaction flask containing the Grignard reagent. A funnel was then placed on the flask and the stirrer switched on. Benzophenone solution was added dropwise to the flask using a dry glass pipette. 5-10ml of ether was used to rinse the beaker of ether, which was then added to the reaction flask. The condenser was then turned on and the reaction mixture gently refluxed for 25minutes. The mixture was allowed to cool to room temperature after which the drying tube and the condenser were removed.
5ml of distilled water was added to dropwise using a glass pipette as the reaction mixture was stirred. This hydrolyzed the alkoxy/magnesium bromide salt to form triphenylmethanol. 15ml of 5% HCL was added with continued stirring until the reaction subsided. The reaction mixture was transferred to a 200ml beaker adding more ether (5-10ml) to remove any residual product. Two layers separated in the beaker.
Product separation
The entire reaction mixture was transferred to a 125ml separatory funnel. The top layer (ether layer) contained the product while the bottom aqueous layer contained the magnesium bromide layer as the by-product. The water layer was drained into the beaker labeled “water layer”. 15ml of 5%aqueous sodium bicarbonate was added to the ether layer in the separatory funnel and shaken vigorously for 1-2minutes.
The mixture separated into water and ether layers on standing and the water layer was drained into the “water layer” beaker. 15ml of saturated sodium chloride was then added to the ether layer and the procedure above repeated. The water layer was drained to the respective beaker while the remaining ether layer was drained into a dry 100ml beaker labeled “ether layer.” 100mg of the drying agent (magnesium sulfate) was then added to the “ether layer” and swirled for some time. The drying agent was filtered off and the solution transferred into a pre-weighed clean and dry 125ml Erlenmeyer flask. Ether was removed from the product by placing the flask in a warm water bath. It was then stored in an open Erlenmeyer flask for one week to dry.
Trituration in Hexane of Crude Triphenylmethanol Product
The solid product was transferred to a 50ml Erlenmeyer flask followed by the addition of 10ml of hexane. The crude triphenylmethanol was gently but repeatedly pressed against the sides of the beaker in hexane (triturated) to promote dissolution of any impurities contaminating the product i.e. purification. The product was collected through vacuum filtration by washing it once with 10ml of hexane in a Buchner funnel. It was then placed on a watch glass to dry.
TLC Analysis of Triphenylmethanol
A TLC plate was prepared and labeled with three tick marks (1, 2, and 3) to analyze the synthesized products (1-benzophenone, 2-product, and 3-known triphenylmethanol). Approximately 10mg of the product was transferred to a vial and 1-2ml of ether was added to dissolve the product. The TLC plate was spotted with the product and solutions of the other known compounds provided. The plate was developed using 90:10 hexane: ethyl acetate. The TLC plate viewed under UV-light and in the iodine chamber, circling the observed spots with a pencil. The Rf values for all the spots observed were calculated.
IR Spectroscopic Analysis of Triphenylmethanol
An IR scan was performed on the product and all major peaks (in cm-1) were recorded for functional group identification.
Weight and Melting Point Determination of Triphenylmethanol
The dry product sample was weighed and the percentage yield was calculated. For melting point determination, the thermometer of the melting point apparatus was first calibrated using benzoic acid (M.p =1220C). The melting point of the product was then determined and recorded. It was compared with the standard melting point of triphenylmethanol to determine its purity.
Work Cited
Ault, Addison. Techniques and experiments of organic chemistry. 6th ed. New York: Prentice Hall, 1998. Print.