Introduction
Decontamination is a very important process at sterile service department because it is meant to ensure that reusable medical instruments do not pose any threat to medical personnel, and patients within a healthcare institution. Schultz and Crow (2008, p. 9) defines decontamination as “The physical or chemical process that renders an inanimate object that may be contaminated with harmful microbial life safe for further handling.” It is the deliberate effort by the department of sterile service to ensure that these instruments are properly sterilized before they can be exposed to other medical personnel to handle them. It is always important to understand the level of risk that each of the instruments pose. These instruments should be categorized based on the level of risk and the approach that should be taken in the process of decontamination. It is important to ensure that any instrument that comes out of this department do not pose any bacterial or viral threat in the orthopedic procedure units or any other units where these instruments are used. Cocciardi (2012, p. 73) says, “The objective of decontamination is to protect the preparation and package workers who come in contact with medical devices after the decontamination process from contracting diseases caused by microorganisms.” It also protects the patients who may be exposed to these contaminated instruments, a fact that may lead to serious infections or re-infections. The recent cases of increased incidences of surgical site infection in the orthopedic procedure unit in this hospital are a clear indication that there is a problem in the sterile service department. This research is expected to unearth this some of the breaches that could have occurred in this department.
Decontamination Process for Reusable Medical Devices
The recent reported of increased incidences of surgical site infection in the orthopedic procedure unit warrants a thorough inspection of the sterile service department. There must be an issue with the decontamination process for reusable medical devices. The process should always ensure that reusable instruments that come out of the department do not pose any threat to the medical staff or patients. It may not be easy to determine where this particular problem could be arising from in this department, unless a detailed investigation is done on all the stages of decontamination within this unit (McDonnell 2007, p. 29). This way, it would be easy to single out some of the procedures at this department that were not carried out as per the specifications.
Collection of used items
The investigation started at the point of collecting used reusable surgical equipments. At this point, it was clear that the attendants responsible for this job were efficient in collecting all the used items at the surgical sites. The reusable items were collected and covered closed totes or plastic bags. The items that could not be used were disposed using the right procedure. It was also observable that these health workers were keen to clean the surgical site in order to ensure that the room was fully sterilized immediately after a surgical process. The reusable items that were to be decontaminated were collected in a covered cart. No breach was detected at this point.
Transport to SSD
The instruments were transported from their collection site to the sterile service department using a covered cart. The carts used at this hospital were manufactured by Medtronic, Inc. It was also noted that before coming out of the surgical site with these instrument, the carts were sprayed with decontaminating chemical to ensure that it does not pose any threat to those who came in contact with it during this movement. These offices also wore protecting clothing as a way of eliminating chances of contamination in the process of carrying out their duties. The investigation could not identify any clear breach at this stage.
Reception
At the sterile service department, the instruments were received by medical personnel responsible for cleaning these items. Each of the instruments had their specific section where they would be placed when they are received. They were classified based on their nature, and the level of cleaning they needed. The sterile service department personnel would take over all other responsibility of sterilizing these instruments once they arrive at this department.
Sorting
Sorting is important to ensure that each instrument is cleaned using the most appropriate method that will eliminate any possible contamination on it. The workers wore appropriate protective clothing before engaging in this activity. At this stage, no clear breach that could be associated to the reported increase in cases of infection at the surgical site was observable.
Cleaning and disinfection
It is important to ensure that these reusable medical equipments are properly disinfected in order to ensure that they do not pose any risk to the patients or medical officers. It involves a series of processes with various machines involved in conducting various tasks. Instruments that were bloody and those with complex design that could not be wiped or rinsed at their point of use were first subjected to rinsing. This was necessary to remove the stains of blood or other debris that could have dried on them. The next stage, which was very important, was the washing of these instruments. Detergent and equipment used at this stage was very important because once the detergent is not compatible with the soil being removed, and then the process cannot be effective. The equipment should have the capacity to wash the instrument thoroughly to ensure that all the debris and other contaminants are completely neutralized. According to Block (2000, p. 46), it is important to ensure that the equipment used in this process passes the set standards. This scholar clarifies that all the equipments must have a CE mark of quality to ensure that the product has the capacity to clean the medical equipment in this department.
After manual washing, the instruments were taken to the ultrasonic washer. The equipment was manufactured by Medtronic, Inc. It was new and very appropriate in eliminating any debris that could have remained in the instrument after washing. It was also observed that at this department, the transport vehicles and equipments were cleaned before they were released out of this unit. The carts were cleaned thoroughly and dried, before they were released back to other units within the hospital. Once again this stage appeared to lack any blemish that could be linked to the increase in incidence of surgical site infection in the orthopedic procedures at this hospital. At this stage, the inspection team thought that the personnel at this unit had known of their mistakes, and were trying to cover any loophole that could expose their irresponsible behavior. However, the investigation continued to other stages.
Inspection
As Schnepp (2010, p. 37) notes, it is important to ensure that all the instruments are subjected to thorough inspection before they can be repackaged for reuse at the hospital. A close analysis on how this process was being conducted revealed some loopholes that could be attributed to the recent cases of infection that was reported. There were only two medical personnel assigned to do this task. When their work was analyzed, it was clear that what they were doing was more of sorting the instruments than inspecting them for any possible threat to other users. They were seen sorting out the instruments into different categories without clearly analyzing how successful the process of cleaning them had been. It was observed that there was no single instrument that was rejected by this group as being unfit for reuse. This was a clear breach that could have led to the incidence of infection.
Assembly
At the assembly point, there were six personnel. The personnel had recipe cards with detailed instructions of how each set of instruments were to be packaged. They were very professional in their work. The instruments were classified into two assemblies, based on their usage. This process had not observable breaches.
Packaging
After assembling the instruments, the packaging stage is very important. The investigation team observed very closely how this process was done in this department. Different types of packaging were used. All the packaging materials used by this unit were manufactured by Medtronic, Inc. The instruments were packaged in four different types of packaging. The four types of packaging included textiles, pouch packaging, nonwovens, and rigid container system. Packaging was done in an environment that was sterilized. This means that the equipments were not exposed to any form of contamination that could be directly associated with the increased incidence of surgical site infection in the orthopedic procedure in the hospital. The activities at this stage were conducted as per stipulated.
Sterilization
According to Fraser and Murray (2003, p. 390), define sterilization as “A process that eliminates or kills all forms of microbial life, including transmissible agents present on a surface, contained in a fluid, in medication, or in a compound such as biological culture media.” In this department, it was observed that sterilization one of the procedures that was given a lot of priority when handling medical equipment. Largent (2011, p. 67) warns that it is important to ensure that all the reusable medical equipment are properly sterilized before they can be used in the surgical sites, or any other department within the hospital. This department conducted sterilization using a variety of approaches based on the nature of the instruments. Some of the methods they applied included use of steam of up to 120 degrees centigrade, ethylene oxide, dry heat, microwave, formaldehyde gas, hydrogen peroxide gas, chemical solutions, and ionizing radiation. The sterilization passed as a standard process based on the duration the instruments were subjected to sterilization agent, and the type of agent chosen for each of the instruments used. There were no observable breaches at this stage.
Storage and distribution
The storage and distribution is another important stage that helps in ensuring that this process is successful (McKeen 2012, p. 83). It is important to ensure that these instruments are stored in an environment that may not expose them to any form of contamination while waiting to be reused. It was observed that the sterile service department of this hospital had a functional sterile storage area where the instruments were kept before they are released into the hospitals. It was observed however, that procedures were not followed by people entering this room. For example, it was common to see these officers trying to handle two trays by hand. Others did not consider using carts to transport these instruments. Their actions were casual, a fact that could lead to contamination of some of these instruments in the store.
Decontamination process controls that involve personnel
Decontamination process controls is also necessary for the personnel. This starts with the attire. Broberg & Roll-Hansen (2005, p. 80) says, “The personnel involved in the decontamination process must have protective clothing that should include scrub uniform that should have moisture-resistance barrier covering, plastic or rubber gloves, hair covering, and shoe covers.” The personnel who are involved in the manual cleaning must wear face masks to protect them from inhaling harmful substances, and safety goggles for eye protection where splashing is involved. It was observed that this was maintained in most of the stages, except in a few cases that have been identified in the above discussion.
Decontamination process controls that involve the environment
The environment within which cleaning takes place, and the surgical site must be subjected to thorough decontamination in order to eliminate any form of contamination that may pose health risk to the personnel or patients who visit the facility. As mentioned before, the surgical sites were cleaned immediately after the procedure. The decontamination sites were also subject to regular cleaning. There were not irregularities reported at this stage.
Decontamination process controls that involve design of the SSD
According to Havill and Boyce (2012, p. 510), the design of sterile service department is important in ensuring that the process of sterilization is successful. The investigation team took time to analyze the design of this unit in order to determine if it conforms to the set standards. It was observed that the design was compliant with the requirements of a standard SSD unit. The reception point of the contaminated materials was on the extreme end of the facility. Each of the cleaning stages described above had its own room that was adjacent to one another until the storage unit that was on the other extreme end, near the surgical site. It was appropriate for this process.
Annotated flow chart
The discussion above has identified all the stages involved in the decontamination of reusable surgical instruments. The annotated flow chart below shows these activities from the time the item is acquired, to the stage when it is disposed out of the cycle. It is important to note that the chart is cyclic because a single instrument may go through the cycle severally before it can be finally disposed.
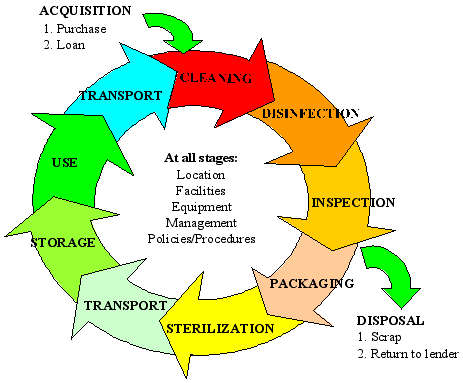
Each of the stages identified above play a pivotal role in ensuring that any form of contamination on the instrument is eliminated completely. As shown in the chart, the process starts with cleaning of the instrument. Any failure to do what is expected at any of the stages may pose serious health consequences to the personnel handling the instruments or the patients.
List of References
Block, S 2000, Disinfection, sterilization, and preservation, Lea & Febiger, Philadelphia.
Broberg, G & Roll-Hansen, N 2005, Eugenics and the welfare state: Sterilization policy in Denmark, Sweden, Norway, and Finland, Michigan State University Press, East Lansing.
Cocciardi, J 2012, Refresher for operating safely in hazardous environments, Jones & Bartlett Learning, Sudbury.
Fraser, V & Murray, P 2003, ‘A Prospective Randomized Trial Comparing Manual and Automated Endoscope Disinfection Methods’, Infection Control and Hospital Epidemiology, vol. 14. no. 7, pp. 383-389.
Havill, N & Boyce, M 2012, ‘Comparison of the Microbiological Efficacy of Hydrogen Peroxide Vapor and Ultraviolet Light Processes for Room Decontamination’, Infection Control and Hospital Epidemiology, vol. 33. no. 5, pp. 507-512.
Largent, M 2011, Breeding contempt: The history of coerced sterilization in the United States, Rutgers University Press, New Brunswick.
McDonnell, G 2007, Antisepsis, disinfection, and sterilization: Types, action, and resistance, ASM Press, Washington.
McKeen, L 2012, The effect of sterilization on plastics and elastomers, William Andrew, Norwich.
Schnepp, R 2010, Hazardous materials: Awareness and operations, Jones and Bartlett Publishers, Sudbury.
Schultz, J & Crow, S 2008, ‘Decontamination Alternative’, Infection Control and Hospital Epidemiology, vol. 11. no. 1, pp. 8-9.