Introduction
Beryl is a mineral composed mainly of beryllium aluminum cyclosilicate. However, traces of cesium, lithium, and sodium can be found in beryl. The gemstone originates from igneous, metamorphic rocks. It has a refractive index ranging from 1.57 to 1.58 and a specific gravity of 2.6 to 2.92 (Sultana & Podila, 2018). Pure beryl is colorless, but it is frequently tinted by impurities hence giving rise to several colors. The most common colors of beryls are colorless, white, blue, green, red, yellow, pink, orange, purple, and brown. Synthetic beryl can be made using methods such as flux-fusion, hydrothermal procedures, and gas transport reaction (Leone, Caucia, Leone, & Marinoni, 2015). Synthetic emerald is made for industrial jewelry purposes. The purpose of this paper is to explain the physical properties of the gemstone beryl in terms of its color-producing mechanisms, crystal structure, and molecular composition, and its uses.

The Color-Producing Mechanism
As mentioned before, pure beryl is colorless. However, the presence of various impurities leads to the production of different colors, hence the diversity of the gemstone. These impurities are chemical elements that are absent in the purest form of the gemstone. Examples include titanium, vanadium, chromium, manganese, iron, copper, and cobalt.
The white or colorless variety of beryl is called goshenite and does not contain any contaminating elements. The deep green variety of beryl is called emerald. It is the most popular and valuable green gemstone. The green color is attributed to the presence of chromium ions (Cr3+). A stone should have a luxuriant, well-defined color in the green to the yellowish-green range. Stones without saturated green colors are referred to as green beryl as opposed instead of emerald. Aquamarine is light blue to blue-green in color and contains iron II as impurities (Fe2+), whereas green beryl contains a mixture of iron II and iron III ions (Fe2+ and Fe3+) (Sultana & Podila, 2018). Heliodor (or golden beryl), which has a yellow to greenish-yellow color gets its hue from iron III ions. Orange and brown beryl are sometimes classified under heliodor. The presence of manganese II ions (Mn2+) results in a pinkish to light purple variant of beryl that is called morganite while manganese III ions as impurities produce red beryl, which is also referred to as Bixbite. Red beryl is a unique, deep red kind that is found in only two places in Utah (Mauthner, 2017).
From these examples, it is obvious that the oxidation states of the impurities determine the resultant color. Therefore, the color of beryl can be altered by changing the oxidation state of the impurities. Aquamarine and morganite usually undergo heat treatment to deepen their colors. On the other hand, heating yellow or green beryl reduces iron II ions to iron II and changes the color of the gemstone to blue, which augments the stone’s monetary value. The other forms of beryl do not undergo additional treatment to alter their color.
Charge transfer is also implicated in color formation in gems such as beryl. However, this process can only take place in gemstones containing a minimum of two elements that differ in their oxidation states. For charge transfer to occur, electrons are exchanged between elements such as Fe2+ and Fe3+ or Mn2+ and Mn3+ (Sultana & Podila, 2018). Additionally, crystals containing mixtures of manganese and iron ions can also participate in charge transfer reactions. Visible light provides energy that leads to the movement of electrons from one ion to another. For example, red light is needed to facilitate the movement of electrons from iron II to iron III and vice versa, which produces aquamarine that appears to the eye as green color because of absorption.
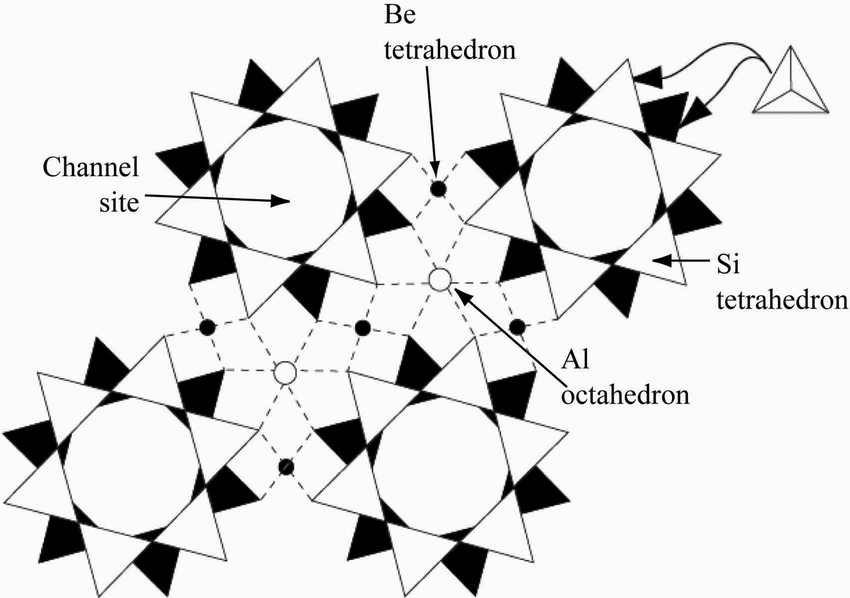
A Diagram of Crystal Structure and Molecular Composition
The chemical formula of beryl is Be3Al2Si6O18. These elements combine naturally to form perfect, prismatic hexagonal (six-sided) crystals of variable sizes. The crystals are very large and have been reported to be as big as 18 meters long and 3.5 meters wide (“Beryl: Mineral information, data, and localities,” 2019). They can take different forms such as stubby or tubular crystals. The bottoms of beryl crystals are often flat. Figure 2 below shows the crystal structure and molecular composition of beryl.
Specific Uses
The main economic use for beryl at present is its use as a gemstone. Its occurrence in a wide range of colors appeals to many people. All variants of beryl can be faceted into different gem cuts and used in jewelry such as earrings, rings, and bracelets (Sultana & Podila, 2018). Emerald and aquamarine are among the most popular jewelry gemstones. Emeralds come second only to diamonds with respect to their dollar value. Despite its beauty, emerald often contains numerous fractures and inclusions. Therefore, there is a need for additional treatment to disguise the breaks and enhance their aesthetic value. Common treatment methods include impregnation with resins or glass to reduce the visibility of the cracks and strengthen the crystal structure. Oiling may also be done for the same purposes. Additionally, processes such as heating and drilling can be used based on the extent of the cracks. Apart from the color richness, aquamarine differs from emerald in structural and surface integrity. Aquamarine has fewer breaks than emerald, which warrants fewer processing steps. The only common treatment for aquamarine is heating to enhance its color. Red beryl is a very rare mineral that is rarely cut into various shapes because collectors value it in its inherent crystal form.
The industrial application of beryl is for the production of the metal beryllium. The stone was previously mined and used to purify the element. Beryllium is a very strong metal that is helpful in the manufacture of alloys where it confers strength to other metals. However, extracting beryllium from beryl is a very costly and labor-intensive process. Therefore, an alternative method is currently preferred where beryllium is produced from bertrandite. Nonetheless, small quantities of the element are still produced from beryl as a byproduct of gemstone mining. The stone is also used in the production of wires and space shuttles (Sultana & Podila, 2018).
Conclusion
Beryl is a gemstone that exists as white or colorless hexagonal crystals in its pure form and variable colors in its impure forms. The color variations are attributed to the presence of specific ions such as chromium, iron, and manganese in different oxidation states. Deep green and blue-green beryls are the most valued versions of the gemstones. Heating alters the oxidation states of impurities and changes the overall color of beryl. Due to its visual appeal, the main use of beryl is in the making of jewelry. Another use of gemstone is the production of the chemical element beryllium. Additional processes such as heating, oiling, and polishing may be required to camouflage flaws and enhance the appearance of the gemstone.
References
Beryl: Mineral information, data, and localities. (2019). Web.
Leone, A., Caucia, F., Leone, A., & Marinoni, L. (2015). AVASPEC 2048: An innovative spectroscopic methodology to differentiate the natural emeralds from the synthetic ones. Periodico di Mineralogia, 84(2), 247-261.
Mauthner, M. (2017). Connoisseur’s choice: Pezzottaite, Sakavalana Mine, Ambatovita, Mandrosonoro Commune, Ambatofinandrahana District, Fianarantsoa Province, Madagascar. Rocks & Minerals, 92(2), 138-143.
McManus, C. E., McMillan, N. J., Harmon, R. S., Whitmore, R. C., De Lucia Jr, F. C., & Miziolek, A. W. (2008). Use of laser induced breakdown spectroscopy in the determination of gem provenance: Beryls. Applied Optics, 47(31), G72-G79.
Sultana, N., & Podila, S. P. (2018). Aquamarine gemstone. IJSRST, 4(1), 342-356.