Introduction
The natural environment contains many micro-organisms that are suspended in the air, in water and on other organisms, for instance, humans. Modern laboratories are busy settings as personnel usually share equipment across overlapping work stations, which are near busy areas or high-traffic instruments. Therefore, it is essential to practice proper aseptic techniques to enable the maintenance of the integrity of cell cultures and a safe working environment. To maintain sterile and safe working surfaces, standard laboratory operating procedures for properly cleaning, disinfecting, and maintaining equipment need to be implemented. Bacteria are among the typical microbiota found in laboratories. The source of bacteria in laboratories includes tap water, bacterial analytical tests, and human contact. Bacteria are nearly found everywhere on Earth as they are capable of growing in diverse conditions. There are many species of bacteria; however, all are unicellular and are capable of rapidly dividing through mitosis. Therefore, under optimum conditions, doubling might occur after every few minutes such that in a few hours, there will be millions of cells from an original single cell. It is also essential to note that some bacteria are harmful, while others are beneficial.
Moreover, some bacteria can be removed from surfaces through cleaning and disinfection; however, others persist over long durations on such surfaces regardless of the utilization of recognized hygienic routines (Møretrø & Langsrud, 2017). Hence when food is dropped onto a surface, any bacteria present on it will adhere to the food. Both food and surface properties affect the rate of transfer of bacteria, and they include surface type, food composition, residence duration of bacteria on the surface, and contact duration of the food with the surface. Contact surfaces tend to acts as reservoirs for bacteria over a long period, and this is because bacteria can survive on such surfaces. The following hypotheses were used to guide the experiment:
- H1 – Bacteria are social microbiota that considerably interact between and within species while responding to external stimuli (Stubbendieck, Vargas-Bautista, & Straight, 2016). Thus this will ground the prediction that several species of bacteria will be present in all plates. As a result, this will enable laboratories to use cleaning methods that control several types of bacteria.
- H2 – Food industries contain residential bacteria; hence, there is a probability that they have been transferred to the bologna (Møretrø & Langsrud, 2017). Conversely, the laboratory sink and benchtop also contain bacteria due to the frequency of human contact (Møretrø & Langsrud, 2017). Therefore, the prediction is that bacteria will be found in both the control and the experimental plates. A comparison of the control and experimental plates enables the experimenter to determine the extent of contamination of the sink and benchtop.
- H3 – The kitchen sink is made of stainless steel, which is a surface type that has been associated with higher bacterial transfer rates, as compared to the benchtop that is made of ceramic tile (Miranda & Schaffner, 2016). Hence it can be predicted that the sink plates will contain a more considerable amount of bacteria that the benchtop plates. Consequentially, will employ different cleaning techniques with regards to the surface type.
- H4 – Nutrient and blood agar are non-selective culture media; hence, they allow for the growth of most bacteria (Lagier, Edouard, Pagnier, Mediannikov, Drancourt, & Raoult, 2015). However, the tetracycline agar is regarded as a selective culture media that permits the growth of specific bacteria. Therefore, the prediction is that bacterial growth will be higher in the nutrient and blood agar as compared to the tetracycline agar. The nature and principles of different agars will enable scientists effectively identify the bacteria species present.
Materials and Methods
Two plates, which are the experimental and control plates, each of nutrient agar, nutrient agar with tetracycline control, and blood agar, were prepared. Bacteria were collected from the bologna deposited on the sink and benchtop. For each new spot, one bologna was used to collect bacteria. The six bologna samples were scrubbed onto the six agar plates. The plates were sealed with parafilm then incubated at 37°C for one week. After incubation, the colonies of the different culture media were observed for Colony Forming Units (CFUs) and recorded based on the shape, size, border, texture, general appearance, and color of individual bacterial colonies on each plate. Refer to the manual for materials and extensive procedures (Pedersen-Shear, Bentley, Frances-Knight, Zeller, & Walters-Conte, 2019).
Results
Colony morphological characteristics
Observations regarding the colony characteristics of the bacteria are presented in Diagram 1 and Table 2. The shapes range from circular to irregular. Additionally, the color varies from white, pink, cream, tan to yellow. The margin of the colonies are generally entire and undulate. Lastly, the colonies are not the same for all three experimental plates, as noted in Tables 3 and 4.
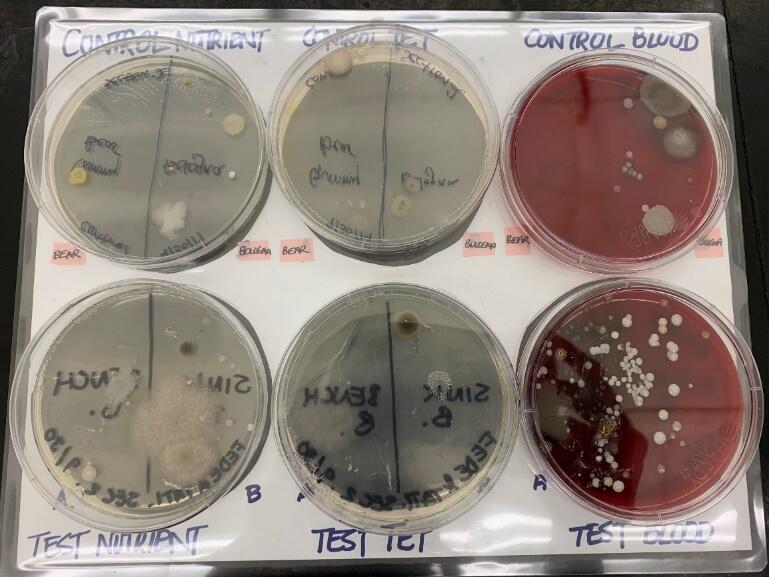
Table 1: Morphology of 8 unique colonies on plates 1-3.
Total number of colonies
Based on Tables 2, 3, and 4, the total number of colonies obtained from the sink and benchtop (+100) is higher than that from the control plates (10). Secondly, in both the control and experimental samples, blood agar exhibits the highest number of total colonies, followed by the nutrient agar, then the nutrient agar with tetracycline control. Thirdly, in reference to Table 4, the sink has more total number of colonies as compared to the benchtop. Colony B is the highest in both the sink and benchtop samples.
Table 2: Total Number of colonies on Bologna Control Plates by TA. (If matching colonies with Table 2 A-H, indicate number present).
Table 3: Total Number of colonies on our Bologna Samples.
Table 4: Blood Agar Plate and Hemolysis.
Beta: most dangerous, ate more blood.
Alpha: Moderate, tried eating away some blood.
Gamma: Less, none.
Discussion
The purpose of the experiment was to compare the presence and degree of bacterial contamination on two food contact surfaces, which are the laboratory sink and the benchtop. It also aimed to investigate the effect of the type of culture media on the growth of bacteria.
The findings of the study supported all four hypotheses. In reference to H1, several species of bacteria were found to be present. The plates had colonies that appeared in varying shapes and colors. The shape varied from irregular to regular with white, cream, yellow, and tan colors. This suggests that colonies are represented by individual bacterial cells that can only be differentiated through sub-culturing. The second hypothesis, H2 was supported in that bacteria were found in both the control and the experimental plates. The presence of bacteria in the control plates can be attributed to cross-contamination while bologna was being processed in the factory. On the other hand, the presence of bacteria in the experimental placed is a result of relatively poor laboratory hygiene.
The results also supported the third hypothesis, H3 – the sink contained a higher amount of bacteria than the benchtop. This is because, based on Table 4, the number of bacteria colonies found on the sinks was twice that on benchtops, which was 16 and 8, respectively. The sink was made of stainless steel which offered facilitated increased bacterial transfer rates than as compared to the benchtop. Moreover, since it is standard laboratory procedure to clean work surfaces, that is, benchtops with disinfectants after use, the benchtop had less harmful bacteria while the sink was characterized to have incredibly harmful bacteria (colony A).
The results of the experiment supported the fourth hypothesis, H4 – bacterial growth will be higher in the nutrient and blood agar as compared to the nutrient agar with tetracycline. In this experiment, three different agars were utilized. In all treatments, the number of colony counts from the nutrient agar, nutrient agar with tetracycline, and blood agar varied, and this illustrated that the type of culture media affects quantitative results. Furthermore, in regards to Table 3, the total number of colonies in nutrient and blood agar was higher than that in nutrient agar with tetracycline. This is attributed to the fact that tetracycline exhibits activity against both gram-positive and gram-negative bacteria. However, the presence of colonies in the nutrient agar with tetracycline suggests the presence of resistant cells in the bologna food sample.
In the experiment, the colonies appeared to be clustered, and only a few were conspicuous. Therefore, this aspect interfered with the counting CFUs using the direct observation approach. Counting of CFUs using the naked eye is a widely used technique in microbiology experiments. However, the CFUs obtained from direct counting is usually considerably lower than the actual number of bacteria present in the media. To determine the direct and exact cell count, microscopy should be used. The variation in the number of plate count cells between the two methods is a phenomenon that is referred to as the “great plate count anomaly.” The reason for the difference in results between direct observation and microscopy can be attributed to the differing nutritional requirements of the bacteria and bacteria growing in large clumps that do not disintegrate when plated.
The characteristics of the “destination” food, that is, bologna, properties that affected the experiment include pH, presence of preservatives, moisture content, and storage conditions (packaging atmosphere and temperature). However, other factors, such as the residence time of bacteria on surfaces, can also affect the experiment. Therefore, if the test is to be repeated, the residence time of bacteria on surfaces should be standardized as it was the only variable that was not constant or pre-determined in the initial experiment.
Nutrient agar, blood agar, and tetracycline agar each have individual and distinct components; therefore, the type of bacteria growing will not be uniform for all plates. For instance, the nutrient agar is suitable for culturing non-fastidious heterotrophic bacteria, the blood agar is ideal for growing heterotrophic bacteria, and tetracycline agar is suitable for resistant bacteria. This phenomenon is also evidence in the results of the findings in Table 3 in which the total number of colonies in each plate was different.
Conclusion
Bacteria from surfaces is transferred to food through contact and in the food industry, this phenomenon can adversely affect food safety quality, and processing. This is because the presence of bacteria can influence the growth and survival of pathogens in such environments. With heightened comprehension of the constituents of residential bacteria and the consequences of such on food safety, food industries need to examine whether particular bacteria are optimal and if resources can be utilized to encourage or restrain the residence of those bacteria. This is because some bacteria are harmful, while others are beneficial. However, regardless of the degree of risk presented by residential bacteria, food industries should embrace strict hygienic measures necessary in controlling pathogens if introduced and to maintain the residential bacteria at a relatively low level. Therefore, changes should be made in control routines, such as cleaning and disinfection, to ensure that the composition of residential bacteria is reduced and kept at a low level. This will lead to the containment the food safety risk.
References
Lagier, J. C., Edouard, S., Pagnier, I., Mediannikov, O., Drancourt, M., & Raoult, D. (2015). Current and past strategies for bacterial culture in clinical microbiology. Clinical microbiology reviews, 28(1), 208–236. Web.
Miranda, R. C., & Schaffner, D. W. (2016). Longer contact times increase cross-contamination of Enterobacter aerogenes from surfaces to food. Applied and Environmental Microbiology, 82(21) 6490-6496. Web.
Møretrø, T., & Langsrud, S. (2017). Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Comprehensive Reviews in Food Science and Food Safety, 16(5), 1022-1041. Web.
Pedersen-Shear, A., Bentley, M., Frances-Knight, S., Zeller, N., & Walters-Conte, K. (2019). BIO-100: Great experiments in biology, lab manual. Web.
Stubbendieck, R. M., Vargas-Bautista, C., & Straight, P. D. (2016). Bacterial communities: Interactions to scale. Frontiers in microbiology, 7, 1234. Web.