Abstract
This case study presents the case of an elderly patient with bradycardia due to a complete AV node block of type III. In this patient, a pacemaker implant is indicated, and the current study evaluates the choice between dual-chamber and single-chamber options. A dual-chamber pacemaker is more appropriate in the absence of atrial fibrillation, but its installation can provoke cases of atrial fibrillation, as was shown in the study. On the other hand, dual-chamber pacemakers have been shown to be more effective and have a better quality of life than single-chamber alternatives. Based on this study, it is recommended that an advanced dual-chamber pacemaker with ventricular control function, adapted for atrial fibrillation, be fitted.
Introduction
The heart muscle is a multi-complex, vital anatomical system which is responsible for the pumping of blood within the body. One of the complexes of the cardiac system is the conduction structures, which ensure the precise coordination of the individual sections of the heart; the atrioventricular (AV) node, located above the coronary sinus opening or directly above the cusps of the tricuspid valve connecting the right ventricle to the right atrium, plays a crucial role in distributing the electrical impulse through the conduction bundles (Figure 1). The AV node, or as it is often referred to in the literature as the Tawara-Aschoff node, receives the signal from the sinoatrial (SA) node located in the right atrial wall and transmits it to the His bundle, whereupon the pulse is distributed along the branches and reaches the subendocardial Purkinje fibres (Aumüller, 2019). In other words, the functional characteristic of the AV node is the mediation of the signal, but just as significantly, the node delays the frequency of the pulse arriving at it, ensuring that ventricular and atrial contractions work together. The numerical value of this delay is well known and is about 0.09 s (Szurgot et al., 2021). If the pulse transmission frequency is maintained or if it increases randomly, the heart is considered to be experiencing a condition called atrial fibrillation (Bailey, 2019). On the other hand, the AV node also has the evolutionarily evolved function of substituting the SA node in the event that the latter’s automatism is disturbed; hence, the AV node is appropriately considered as a second-order automatism centre, which nevertheless has an effect on heart rate. In other words, the functional importance of the AV node is undeniable, as the existence of such a node in the cardiac conduction system resolves issues related to the coordination of ventricular-atrial contractions and the uniform distribution of electrical signals across the following bundles.
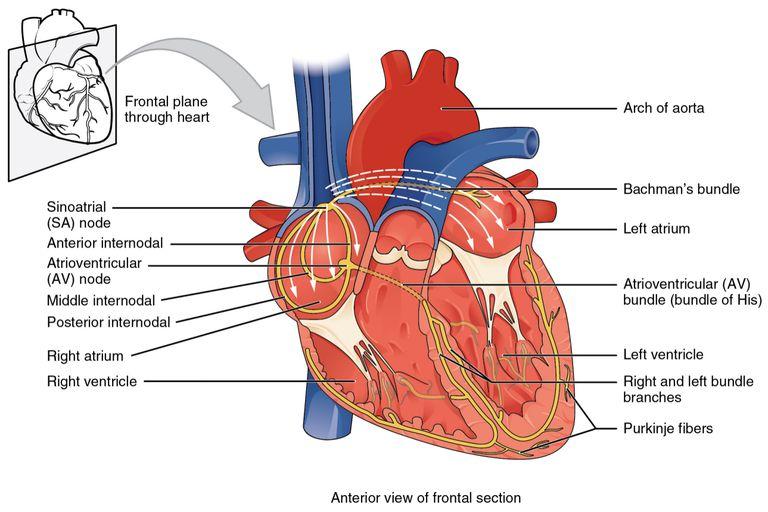
The normal function of the AV node can be impaired, with both congenital and acquired factors being the cause of such abnormalities. In the case of congenital abnormalities, autoimmune diseases producing autoantibodies — such antibodies penetrate the placental barrier of the developing foetus and destruct the normal development of cardiac tissue, causing the formation of replacement scars (Limaye et al., 2020). On the other hand, abnormalities of the AV node may be due to acquired causes, among which extracardiac and cardiac factors must be distinguished. As a result of extracardiac factors disturbing the natural range of cardiac contraction, the AV node experiences stress resulting in a decline in functionality: such factors should include excessive physical exertion, electric shocks, and disruption of the parasympathetic nervous system. Cardiac factors include any diseases related to cardiac performance: these include coronary heart disease, cancer, physical injuries and heart attacks. Among other things, the performance of the AV node is affected by the associated SA node, which for a variety of reasons may generate pulses of a lower input frequency than the output frequency of the AV node — in which case there is an occurrence recorded with a negative P wave on the electrocardiogram (Chang, Wang, and Jin, 2022). However, it is not always clinically possible to identify the pathology of impaired pulse microcirculation, and studies have tended to generalise the totality of such conditions by several terms, among them Lenegre’s disease, primary cardiac block or idiopathic AV block (Bhat, 2019). Regardless of the cause, the AV node of the conduction system appears defective, which directly affects the functionality of the heart.
If the electrical impulse is not conducted to the ventricle or the delay is abnormal, this condition is known as an atrioventricular block. Three types of a block are distinguished depending on what pattern is seen on the electrocardiogram (Wiginton, 2022). In fact, a type I blockade is not a complete blockade but reflects a delayed signal, resulting in an increase in heart rate. A type II blockade is realised when part of the atrial pulses does not reach the ventricles; remarkably, two subtypes of this type of blockage are distinguished depending on the change in the P-Q interval. In Mobitz I blockade, the P-Q interval is prolonged and followed by a single P wave, whereas in Mobitz II blockade, there is a QRS complex dropout without a change in P-Q interval length. Finally, type III blockade also called complete blockade, prevents ventricular impulses from reaching the ventricles due to the dysfunctional AV node: ventricular contraction decreases, but the atrial contraction rate remains intact.
AV node dysfunction is treated surgically by implanting a pacemaker into the chest area. This device automatically regulates the inhibited electrical impulses delivered to the ventricles by generating signals of selected frequencies to replace the AV node. The pacemaker is not inserted directly into the heart or replaces the pumping function of the muscle but regulates the transmission of electrical impulses in the cardiac conduction system by means of electrodes placed in the heart chambers. Although the practice is time-tested and scientifically proven, it does not rule out the formation of complications. These include infections caused by pathogen invasion, mechanical damage to the pacemaker and displacement of electrode placement, and venous thrombosis (Iwata et al., 2021). These complications require surgical clinical intervention to restore substituted AV node function. The following sections suggest a discussion of the applicability of cardiac stimulation to the bradycardia patient.
Case Data
Patient aged 81 years old male. History of hypertension: symptoms of dizzy spells was admitted to A& E the ECG showed complete heart block which is class 1 indication for pacing (according to AHA/ACC guidelines) patient was implanted with a dual chamber pacemaker (DDD) on 8th October 2021. Post implant assessment showed all test results within normal limits. Patient went into Atrial flutter during implant. Ventricular auto capture programme on. Home monitor issued. Pacemaker was programmed to VVI mode due to atrial flutter. On 29 November 2021 patient attended for face to face follow up due to low R wave sensing note via home monitoring. ECG showed atrial flutter with appropriate ventricular pacing. Manual test results showed R wave of 2.8-3.6mV. Patients underlying heart rate is 42bpm. Patient was 99% V paced. An elective RV lead reposition was conducted( complication of early lead displacement can be discussed here). After lead reposition on 11/01/2022 patient was again seen in face to face pacemaker clinic due to an out of range RV lead impedance on 25/01/2022. No RV capture in bipolar at maximum output and no RVsensing in bipolar.the pace sense configuration remains unipolar. Unipolar ventricular pacing at 62bpm. Underlying complete heart block with a ventricle rate of 35bpm. Patient also complain of a twitch in the pacemaker pocket. ( complication).patient was booked for an RV lead replacement due to low lead impedance, consistent with lead insulation breach. It is believed that there may have been some damaged caused to the RV lead during repositioning of RV lead procedure. Post lead replacement checks showed lead impedance of 700 ohms. V Threshold of 1.2 at 0.4ms. Sensing 5mV.
Discussion
The present case study examines the case of a patient with bradycardia who was initially fitted with a dual-chamber pacemaker replacing the AV node function. Firstly, it should be clarified that permanent pacemakers come in two variants depending on the number of electrodes used. A single-chamber (VVI) pacemaker electrode is placed in the lower cavity of the right ventricle — it is not the only option — this solution covers the problem of bradycardic contraction of the atrial fibres, in which the right atrium has virtually no functionality (Cho, 2020). The placement of a VVI pacemaker directs impulses directly to the right ventricle, replacing the work of the blocked AV node and allowing blood to push out normally into the arteries during ventricular contraction. In contrast, dual-chamber pacemakers (DDD) have two electrodes, one of which is placed in the right atrial cavity and the other in the right ventricular cavity. The mechanism of action of such pacemakers aims to generate an electrical impulse in the right atrium, motivating both atria to contract, after which a signal is sent to the second electrode, which excites ventricular contraction (Cho, 2020). In other words, DDD pacemakers completely replace the cardiac conduction system provided that there is no atrial fibrillation and, therefore, the pulse to the atria is sufficient to stimulate atrial contraction. In contrast, if the atria experience atrial flutter, the installation of a dual-chamber pacemaker proves impractical, as it may not lead to the desired clinical effects compared to a VVI (NICE, 2014). It is necessary, however, to investigate which mode of pacemaker is most effective in cases of complete AV blockade, as observed in the patient.
During complete type III blockade, atrial fibrillation is usually not seen, so DDD is the most recommended choice. In this case, the atria are functioning normally — except for damage to the AV node — so are able to pick up an artificially generated pulse at the first electrode, followed by their contraction. The second signal is sent to the ventricular electrodes, which in turn excites them to contract and push blood out into the artery. AV synchrony is then maintained, which increases the autonomy and coordination of the micro regulated pulse system (Panicker, Desai, and Lokhandwala, 2009). The case of the elderly patient is no exception, so following an electrocardiogram observation after the patient was diagnosed with complete AV blockade, it was decided to insert a DDD type pacemaker as the man’s atria did not initially show fibrillation. This coincides with academically known practice: DDD pacemakers have been found to improve the quality of life for patients with AV blockage compared to the use of VVI, with which there is an increase in NT-proBNP concentration diagnosed in the development of heart failure (Santana et al., 2019; Zhang et al., 2018). Among others, VVI stimulator use in the absence of suspected atrial fibrillation has been reported to increase rehospitalisation among patients compared with DDD (Sasaki et al., 2022). When R wave activity persists in patients with AV block, researchers suggest implantation of DDD because it is less likely to cause adverse effects (Yilmaz, Akcay and Yesil, 2022). Moreover, according to the ACC/AHA guidelines, DDD is preferable for type III blockade, as a review of several RCTs has shown that DDD reduces the likelihood of atrial fibrillation syndrome (Kusumoto et al., 2019). In addition, an analysis of randomised trials by NICE also showed that according to 4.1.2-4.1.9, dual-chamber stimulation led to a statistically significant reduction in the risk of atrial fibrillation (NICE, 2014). In other words, the choice of DDD stimulator for the case study was more appropriate based on the results of the primary diagnosis according to the reviewed literature. The collected diagnostic findings of the elderly patient after implantation showed a positive trend, from which it can be concluded that DDD was correctly chosen.
On the other hand, the patient’s history shows a low R-wave electrocardiogram some time after the DDD was implanted, suggesting abnormal atrial function. The atrial fibrillation detected was an indication that the DDD stimulator was not working correctly, so the mode was changed to VVI. The detected effects have long been investigated in the literature; for example, Fröhlig et al. (1985) reported that DDD stimulation caused atrial fibrillation in patients even when there was no suspicion prior to implantation. Schuchert and Meinertz (1998) reported that patients might develop atrial fibrillation in 3-6% of cases if DDD stimulators did not have automatic mode switching. During a five-year follow-up, it was also found that 10.4% of patients with DDD were at risk of developing fibrillation in the absence of symptoms prior to implantation (Campos et al., 2017). Among other things, NICE (2014) highlighted that the use of DDD increased the likelihood of fibrillation compared to VVI, but the difference was not statistically significant. Apparently, the case of the patient with bradycardia belongs to those exceptions where DDD implantation causes undesirable effects in the form of atrial fibrillation.
In other words, DDD implantation can lead to adverse effects, as suggested by a review of the scientific literature. Other complications from the use of dual-chamber stimulators include the development of AV dyssynchrony, in which there is an adverse delay between atrial and ventricular contractions (Biffi et al., 2021). In addition, the choice of outdated DDD stimulators with polyurethane isolation and monopolar design led to a deterioration of patients in a retrospective study (Dębski et al., 2018). Evidence-based complications also include right ventricular wall perforation causing pneumothorax and cyst formation (Iwata et al., 2021). In other words, DDD stimulators do not exclude the possibility of complications caused both by choice of poor-quality materials and negligence during implantation, as well as by the physiological consequences of the dual-chamber stimulator. Consequently, the use of DDD can be life-threatening due to these complications, and the implantation of more advanced devices is necessary.
Such devices include the DDD-MVP, a new generation DDD stimulator that provides ventricular stimulation control. The DDD-MVP has been reported to show increased efficacy even in atrial fibrillation, which is particularly relevant to the clinical case studied (Sweeney et al., 2005). The device automatically adapts to the atrial contraction and adjusts between modes to ensure optimal haemodynamics. This solves the problem of unwanted atrial fibrillation by reducing the amount of stimulation; in other words, the pacemaker works on demand.
Conclusion
For the patient in the case study, implantation of a DDD pacemaker has led to undesirable consequences, including atrial fibrillation. The development of this condition required the pacemaker to be switched to VVI mode, which was performed according to the treatment history. Based on the data collected and the literature reviewed, an advanced dual-chamber pacemaker, the DDD-MVP, with ventricular stimulation control is the best choice for the patient: such a device is suitable for atrial fibrillation and allows automatic switching between modes to adapt to the heart response. The DDD-MVP covers the complications that can result from using a DDD pacemaker and maintains AV synchronisation of cardiac structures.
Reference List
Aumüller, G. (2019) ‘The discovery of the cardiac atrioventricular node by Sunao Tawara and Ludwig Aschoff,’ Deutsche Medizinische Wochenschrift (1946), 144(25), pp. 1771-1777.
Bailey, R. (2019) Heart nodes and electrical conduction. Web.
Bhat, R. (2019) ‘Electrocardiographic diagnosis: ‘eegular block’,’ APIK Journal of Internal Medicine, 7(4), pp. 141-141.
Biffi, M., Spadotto, A., Piemontese, G.P., Toniolo, S., Bartoli, L., Sorrentino, S., Minguzzi, A., Massaro, G., Capobianco, C. and Statuto, G. (2021) ‘Cardiac stimulation in the third millennium: where do we head from here,’ Hearts, 2(1), pp. 15-35.
Campos, N.L.K.L.D., Andrade, R.R.D., Fellicio, M.L., Martins, A.S., Garzesi, A.M., Garcia, L.R. and Takeda, T.B. (2017) ‘Comparative study of electrical stimulation of the heart with VDD and DDD pacemakers as to the evolution to atrial fibrillation,’ Brazilian Journal of Cardiovascular Surgery, 32, pp. 347-353.
Chang, Q., Wang, G. and Jin, Y. (2022) ‘Digging Deeper for the differential diagnosis of negative p waves in lead i—reply,’ JAMA Internal Medicine, 182(3), pp. 358-359.
Cho, J. (2020) DDD is superior to VVI? Web.
Dębski, M., Ulman, M., Ząbek, A., Boczar, K., Haberka, K., Kuniewicz, M., Lelakowski, J. and Małecka, B. (2018) ‘Lead-related complications after DDD pacemaker implantation,’ Kardiologia Polska, 76(8), pp. 1224-1231.
Fröhlig, G., Sen, S., Rettig, G., Schieffer, H. and Bette, L. (1985) ‘Atrial flutter and atrial fibrillation by DDD stimulation,’ Zeitschrift fur Kardiologie, 74(9), pp. 537-547.
Iwata, S., Hirose, A., Furui, I., Matsumoto, T., Ozaki, M. and Nagasaka, Y. (2021) ‘Right ventricular perforation, pneumothorax, and a pneumatocele by a pacemaker lead: a case report,’ JA Clinical Reports, 7(1), pp. 1-4.
Kusumoto, F.M., Schoenfeld, M.H., Barrett, C., Edgerton, J.R., Ellenbogen, K.A., Gold, M.R., Goldschlager, N.F., Hamilton, R.M., Joglar, J.A., Kim, R.J. and Lee, R. (2019) ‘2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society.’ Journal of the American College of Cardiology, 74(7), pp.e51-e156.
Limaye, M.A., Buyon, J.P., Cuneo, B.F. and Mehta‐Lee, S.S. (2020) ‘A review of fetal and neonatal consequences of maternal systemic lupus erythematosus,’ Prenatal Diagnosis, 40(9), pp. 1066-1076.
NICE (2005) ‘Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block,’ Technology Appraisal, 88, pp. 1-38.
Panicker, G.K., Desai, B. and Lokhandwala, Y. (2009) ‘Choosing pacemakers appropriately,’ Heart Asia, 1(1), pp. 26-30.
Santana, D.R., Santana Filho, G.P., Rocha, Z.B., Lima, A.M.C., Nery, M.W., Rassi, S. and Gardenghi, G. (2019) ‘Impact of DDD and VVIR stimulation modes on functional capacity and quality of life of chagasic patients,’ Journal of Cardiac Arrhythmias, 32(1), pp. 30-37.
Sasaki, K., Togashi, D., Nakajima, I., Suchi, T., Nakayama, Y., Harada, T. and Akashi, Y.J. (2022) ‘clinical outcomes of non-atrial fibrillation bradyarrhythmias treated with a ventricular demand leadless pacemaker compared with an atrioventricular synchronous transvenous pacemaker―a propensity score-matched analysis,’ Circulation Journal, pp. 1-9.
Schuchert, A. and Meinertz, T. (1998) ‘Pacemaker therapy in patients with atrial fibrillation,’ Herz, 23(4), pp. 260-268.
Sweeney, M.O., Ellenbogen, K.A., Casavant, D., Betzold, R., Sheldon, T., Tang, F., Mueller, M., Lingle, J. and Marquis MVP Download Investigators (2005) ‘Multicenter, prospective, randomized safety and efficacy study of a new atrial‐based managed ventricular pacing mode (MVP) in dual chamber ICDs,’ Journal of cardiovascular Electrophysiology, 16(8), pp. 811-817.
Szurgot, M., Wieczorek, W. and Grabarek, B.O. (2021) ‘Interpretation of ECG recordings: part,’ Medical Science, 15, pp. 55-61.
Wiginton, K. (2022) What is atrioventricular (AV) block. Web.
Yilmaz, R., Akcay, F.A. and Yesil, M. (2022) ‘The effects of pacing mode on development of chronic AF in patients undergoing pacemaker implantation due to AV block: a retrospective study,’ Medicine, 11(1), pp. 48-52.
Zhang, X., Li, Y., Wang, N., Zhang, C., Zhang, D. and Li, Q. (2018) ‘Effects of permanent cardiac pacemaker implantation on vascular endothelial function, blood coagulation and cardiac function in patients with bradycardia,’ Experimental and Therapeutic Medicine, 16(6), pp. 4717-4721.