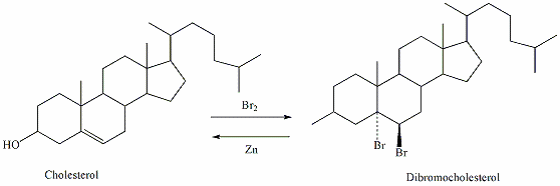
Bromination/debromination which is an important organic reaction that aims in purification of crude cholesterol from impurities which include 3-cholestanol, 7-cholesten-3-ol, and 5,7-chlestadien-3-ol was performed in a laboratory scale for two weeks. Due to steric constraints, only cholesterol reacted with bromine and crystallized from the solution making it possible to be separated (Feiser, and Williamson 63).
The dibromocholesterol formed is regenerated by reacting with Zinc dust. In addition, three methods were used to evaluate the effectiveness. These were Sodium Iodide test, Silver Nitrate test, and Sulfuric acid test. NaI test showed a positive response as color changed to yellow. The formation of the precipitate also indicated a positive result. The sodium iodide reagent reacted with 1° and 2° alkyl halides through an SN2 mechanism. On the other hand, the silver nitrate reagent reacted with 2o and 3° alkyl halides through an SN1 mechanism. Negative results were observed for both the commercial cholesterol and 1-chlorobutane (Zubrick 38).
Conversely, the t-butyl chloride gave a positive result for the AgNO3 test and a negative result for the NaI test. The synthesized cholesterol was 0.29 gram and the theoretical yield was 1.08 gram. This gave a percent yield of 26.9 gram. Although this was a low yield the TLC analysis confirmed a high purity of the synthesized cholesterol. The melting point of the synthesized cholesterol and commercial cholesterol seems to fall in the same range. This confirms the purity of the synthesized cholesterol. TLC analysis was carried out to confirm the purity of the analysis. The distance traveled by commercial cholesterol was 5.5 whereas that travelled by the synthesized cholesterol was 4.6. The absence of other spots on the TLC plate confirms that there were no contaminations present in the sample.
Introduction
Cholesterol is an important steroidal compound (a combination of steroid and alcohol) found in both animals and plants. In animals it is transported in the blood plasma. Larger amounts of cholesterol are found in the cell membranes of all body tissues. Despite the fact that cholesterol causes cardiovascular diseases, it plays a vital role in life. For example, cholesterol is the main structural component in cell walls and in myelin sheath formation of the nerve tissues. It is also a major precursor for most steroid hormones. Cholesterol in internally synthesized in the body in parts with densely packed membranes such as the brain, spinal cord, and liver, among others.
The molecular formula of cholesterol is C27H46O with a molecular mass of 386.65 gram/mol. The melting point of pure cholesterol has been cited to range from 146-1470C. Crude cholesterol is isolated from natural sources and various methods have been used in its purification. Crude cholesterol contains approximately 3-5% contamination. Some of the contaminants are 3-cholestanol, 7-cholesten-3-ol, and 5, 7- chlestadien-3-ol shown below.
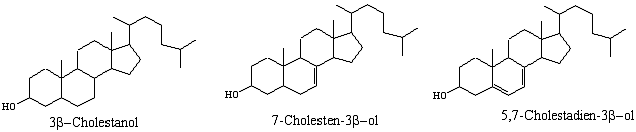
The main objective of this experiment was to purify commercial cholesterol using organic reaction chemistry, including the use of the electrophilic addition. For complete purification of cholesterol from the above impurities is achieved by a reaction of bromine with cholesterol to generate dibromocholesterol. Because of the steroid ring structure present in these compounds that causes steric constraints, only cholesterol reacts with bromine to form an insoluble diaxial dibromo compound through electrophilic addition. On the other hand, cholestanal does not react with bromine and the other two contaminants are dehydrogenated by bromine leading to formation of soluble dienes and trienes respectively. The dibromo-cholesterol precipitates as a solid leaving the other impurities in the reaction solvent.
A purification step such as solvent washing or crystallization is carried out to separate the solid from the impurities. The solid dibromo-cholesterol is then reacted with zinc in order to regenerate pure cholesterol.
To test the effectiveness of this reaction three different chemical tests namely sodium iodide in acetone, silver nitrate in ethanol test, and sulfuric acid test, were performed. Each of these tests is selective for a specific functional group. Dibromocholesterol contains both primary and secondary alkyl halides and reacts with a sodium iodide in acetone and silver nitrate in ethanol to form a precipitate or a cloudy solution. In addition, the presence of double bonds in dibromocholesterol in form of alkene makes it possible for the formation of a fluorescent green sulfuric acid layer and a red chloroform layer when reacted with sulfuric acid (Landgrebe 78).
Material and Methods
1g of commercial cholesterol was added to a 25 mL Erlenmeyer flask. 7 mL t-butylmethyl ether was measured with a graduated cylinder and added to the flask containing the cholesterol and a magnetic stir bar. A water bath was then set up on the hotplate in the hood. The Erlenmeyer flask contain the reaction solution was inserted into the water bath and clamped as shown below. The heat and the stirrer were turned on and gently heat until all the cholesterol dissolved in t-butylmethyl ether.
The flask was removed from the water bath after all the cholesterol was completely dissolved and allowed to cool to room temperature. After the cholesterol solution was cooled, the flask was clamped to the ring on the hot plate as shown in figure 4 and stirred without heating. A burette was then used to dispense 5 mL of bromine solution into the flask. A precipitate solution formed almost immediately.
The water bath was replaced with ice and tap water and the reaction solution stirred intermittently with a glass stir rod for ~ 10 minutes to complete the crystallization of the product. About 20 mL of the t-butylmethyl ether – acetic acid solution was then dispensed in a clean 50 mL Erlenmeyer flask which was clamped to a ring stand and allowed to cool in the ice bath. A vacuum filtration was done using a Buchner funnel and filter paper.
The solid in the filter was washed using ~10 mL of the cooled solution of t-butylmethyl ether – acetic acid and then with ~10 ml of methanol. The solid was then allowed to dry with the vacuum on for about 5 minutes. The dibromocholesterol weight was measured and recorded. In addition the melting point test was done by dropping a capillary filled with 2-4mm of the dibromocholesterol in a bounce tube. The capillary was then placed into a slot in the Mel-Temp when the device is turned on and the fan is unplugged. The heating dial was turned to a setting to 3 and then gradually increased.
The dibromocholesterol sample was seen through the sample viewer and the melting point range of the sample was recorded using the thermocouple. The melting point was recorded when the crystal began to melt and recorded again when the crystal completely melted into liquid. Then the cooling fan was plugged in. The actual melting temperature was obtained from a text book. The experimental melting point and the actual melting point were compared.
The dry solid was weighted and sealed in a vial and stored for next experiment. To debrominate cholesterol, 20 mL of t-butylmethyl ether, 5 mL of acetic acid and 0.2 g of Zn dust were added into the Erlenmeyer flask containing the dibromocholesterol solid. The mixture was swirled for 5-10 minutes in the hood and sonicated in 5 minutes to allow the reaction to go completion. After sonication the solids present were removed by gravity filtration method into a clean 125 mL Erlenmeyer flask. The filtrate was transferred to a 125 mL separatory funnel in which 20 mL of deionized water was added, shaken and allowed to separate into layers.
The two layers formed were then separated as water layers and organic (ether) layers. The ether layer was washed with 20 mL of 10% NaOH and then 20 mL of saturated NaCl solution. 100mg of the drying agent magnesium sulfate was added to the organic layer and the solution swirled until dry. The drying agent was removed by gravity filtration using a glass funnel fluted filter paper and a very dry 50 mL Erlenmeyer flask.
The flask was placed in a warm water bath and then ice cooled for 10 minutes until all but 5 mL of the ether remained following a precipitate formation from the solution. The remaining solvent was decanted and the synthesized cholesterol transferred and allowed to dry in the hood for 20 minutes.The dry solid was weighed and the weight recorded. The melting point of the regenerated cholesterol was measured as described above in the measurement of melting point for the dibromocholesterol.
To evaluate the effectiveness of the bromination reaction three chemical reactions mentioned above were carried out.
NaI in acetone test
Five test tubes labeled A, B, C, D and E were used for this test. About 30 mg of the commercial cholesterol starting material was added to tube A; ~30 mg of dibromocholesterol to tube B; ~30 mg of the synthesized cholesterol product to tube C; ~0.3 mL of 1-chlorobutane to tube D; and ~ 0.3 mL of t-butyl chloride to tube E. In addition, about 3 mL of acetone was added to each tube to completely dissolve all the compounds. Solutions A-E was used to do the NaI in acetone test as well as the AgNO3 in ethanol test. Tubes A-C did the TLC as well. The NaI in Acetone and AgNO3 in Ethanol tests were performed by setting up a test tube rack containing ten small test tubes.
The test tubes were labeled N1 – N5 and A1 – A5. 1 mL of NaI in acetone reagent was added to test tubes N1 – N5, and 1 mL of AgNO3 in ethanol reagent to test tubes A1 – A5. This was followed by adding 5-8 drops of A solution to test tube N1 and tube A1, 5-8 drops of solution B to test tube N2 and tube A2, 5-8 drops of solution C to to test tube N3 and tube A3, 5-8 drops of solution C to test tube N4 and tube A4, and 5-8 drops of solution C to test tube N5 and tube A5.
The test tubes were heated for a while and all the observations recorded. The sulfuric acid for alkenes test was performed by additional solutions of cholesterol and dibromocholesterol with five dry-cleaned test tubes 1-5. 10 mg of commercial cholesterol was placed in tube 1, ~10mg of the dibromocholesterol to tube 2 ~10 mg of your synthesized cholesterol to tube 3, ~10 mg of 2-chlorobutane to tube 4, and 10 mg of cyclohexene to tube 5. About 1 mL of chloroform (CHCl3) was added to each tube and vortex to completely dissolve all solids. In addition, 0.5 mL of H2SO4 was then added to each tube. The observation for this reaction was recorded in the notebook.
The TLC analysis of cholesterol and dibromocholesterol was performed by obtaining a silica gel TLC plate and setting it up to run TLC analysis on solutions A-C above. The plate was spotted with each solution and developed by placing the plate using 30% ethyl acetate: 70% hexane as the mobile phase. The spots were allowed to dry before continuing with the experiment. In addition, the solvent was allowed to rise to within a finger’s width of the top of the paper. The developed plates were viewed under UV lamp and in the I2 chamber and observations recorded.
Results
Table 1 Percent Yield of synthesized cholesterol.
Mass of Synthesized Dibromocholesterol was 1.57g
Table 2 Melting Point Test.
Table 3 Sodium Iodide test.
Table 4 Silver Nitrate test.
Table 5: Sulfuric Acid Test.
Table 6: Thin Layer Chromatography: In 30% Ethyl Acetate/70% Hexane.
Discussion
The percent yield is the ratio of the experimental or actual yield to the theoretical yield. In this experiment, the mass of the synthesized cholesterol was.29 grams (Table 1). The theoretical yield was determined as 1.08 grams. The actual yield was calculated by taking the difference of the weight of the round bottom flask and the synthesized cholesterol by the synthesized cholesterol’s weight alone. The percent yield was calculated as actual yield divided by theoretical yield. The ratio was multiplied by 100 to give 26.9 percent as the percent yield. The bromination/debromination of cholesterol seems inefficient in our case because of the low yield and percent yield of the synthesized cholesterol.
The melting point of the synthesized cholesterol agrees with the melting point of the commercial cholesterol since they fall within the same range. That is commercial cholesterol has a melting point that ranges between 144.6-150.3 degrees centigrade whereas that of the synthesized cholesterol was measured as 147 degrees centigrade. Because of this agreement in melting point, it was confirms the purity of the synthesized cholesterol. In addition this means that, the synthesized cholesterol had no impurities or contaminants.
NaI test showed a positive response as color changed to yellow for the dibromocholesterol. In addition, synthesized cholesterol also gave a positive result with sodium iodide test as the solution turned in the formation of chunky and yellow solution. This may be due to the presence of small amounts of primary or secondary alkyl halides still present in the synthesized compound. On the other hand, a negative result was recorded with commercial cholesterol. This is because commercial cholesterol does not have primary or secondary alkyl halides. The reaction of NaI with 1-chlorobutane resulted in yellowish coloration whereas that with tert-butyl chloride a cloudiness solution was observed.
The formation of the precipitate also indicated a positive result. The sodium iodide reagent reacted with 1° and 2° alkyl halides through an SN2 mechanism. On the other hand, the silver nitrate reagent reacted with 2o and 3° alkyl halides through an SN1 mechanism. Negative results were observed for both the commercial cholesterol and 1-chlorobutane. This is because of lack of 2o and 3° alkyl halides in these two compounds. Conversely, the t-butyl chloride, synthesized cholesterol and dibromocholesterol gave a positive result for the AgNO3 test.
The sulfuric acid test was also carried to evaluate the effectiveness of the bromination/debromination of cholesterol. Theoretically and also experimentally, sulfuric acid reacts with molecules containing alkene functional group give a positive observation of color change and temperature change. In this experiment, there was a rapid evolution of heat and change in color that indicated a positive reaction with commercial cholesterol, dibromocholesterol, synthesized cholesterol, and tert-butyl chloride.
On the other hand, no color change was observed between the reactions of 1-bromobutane with sulfuric acid. This is obvious since, 1-bromobutane does not contain alkene functional group. The stationary phase of the TLC test was the silica gel TLC plate and the mobile phase was 30% Ethyl Acetate/70% Hexane (Table 6). The mobile phase carries the components of the sample. The leading edge of the solvent is known as the solvent front. Each component is preferentially attracted to either the stationary phase (TLC plate) or the mobile phase (solvent) by intramolecular attractions. Thus due to differences in their molecular structures, the components moved up the paper at different rates. This caused them to separate. The components are characterized by their retention factor (Rf).
Retention factor is the ratio of the distance travelled by the component and the distance travelled by the solvent front. The distance traveled by commercial cholesterol was 5.5 resulting in a retention factor of 1. This confirmed the high purity of commercial cholesterol. The synthesized cholesterol spot travelled a distance of 4.6 with a retention factor of 0.84. This was in good agreement with the commercial cholesterol and confirms lesser impurities.
The dibromocholesterol spot travelled a distance of 4 resulting in 0.73 retention time. This is because of the presence of bromine this compound. The difference in the distance traveled and the retention factors values of the commercial and synthesized cholesterol were pure with synthesized having minimal impurities. Since there were no other spots visible on the TLC plate was a clear indication that there were no contaminations of other chemical compounds present in the sample. In addition, good laboratory techniques contributed in achieving good separation of the components.
References
Feiser, Louis, and Williamson, Kenneth. Organic Experiments, 8th Edition, Houghton Mifflin Co.: New York, 1998. Print.
Landgrebe, John. Theory and Practice in the Organic Chemistry Laboratory, 4th edition, Brooks/Cole Publishing Co.: Pacific Grove, CA, 1993. Print.
Zubrick, Jams. The Organic Chem Lab Survival Manual, 4th Edition, John Wiley & Sons: New York, 1997. Print.