Chemistry of Yoghurt
Yogurt is a product of fermentation, a process through which sugar molecules are broken down in an anaerobic (without oxygen) environment aided by microorganisms such as bacteria, fungi, and viruses to form carbon dioxide and acid or alcohol. In yogurt, lactic acid in milk is fermented by bacteria, specifically Streptococcus and Lactobacillus species, to form lactic acid.


When the bacteria ferment the sugar lactose into lactic acid, this acid causes the proteins in milk to coagulate. As lactic acid accumulates in milk, it decreases the pH level, causing the milk to clot and form a soft gel. The yogurt flavor is due to the lactose that remains in milk after coagulation. Lactic acid is sour, thus the taste of yogurt and kimchi.
Chemistry in Batteries
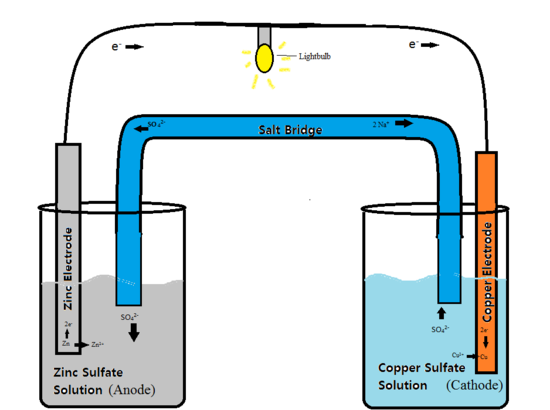
Batteries are devices that store chemical energy and then convert it to electrical energy to power machines, gadgets, equipment, and systems. This chemistry is called electrochemistry, and the process of converting chemicals into electrical energy is electrochemistry. The single unit that completes this function is the electrochemical cell. A battery may have many electrochemical cells that convert chemical to electrical energy. Each of these cells has two electrodes and an electrolyte separating them- a positive electrode (anode) and a negative one (cathode) (Rybolt, 2019). Electrodes are pieces of metal such as zinc and copper that can react with a chemical (electrolyte) such as soluble salts.
One metal reacts with the electrolyte and releases electrons that have negative charges and are attracted by the other metal with positive charges. Electrons flow from the anode (negative electrode) to the cathode (positive charges). When the two metals are connected, they complete an electrical circuit.
Reference
Rybolt, T. R. (2019). Chemistry in your everyday life. Enslow Publishing, LLC.