Literature Review
The perception of color arises when light receptors in the eye convey messages to the brain, hence producing different sensations of color. Early observations by Newton revealed that color is not inherent in objects.1 Instead, the surface of an object reflects certain colors and absorbs others. Therefore, the eyes only perceive the reflected colors. The precise wavelengths that are absorbed or reflected depend on the attributes of the object. Many compounds take in ultraviolet (UV) rays, which are also referred to as visible light. The amount of light absorbed may be measured as transmittance or absorbance.2 On the one hand, absorbance is inversely proportional to transmittance. Therefore, if the light passes through a solution devoid of any absorption, net absorbance is zero, whereas transmittance is 100%.3 On the other hand, percent transmittance is zero if all the light is absorbed. This relationship can be explained by Beer Lambert’s law which states that A=ebc where A is absorbance, e is the molar absorptivity measured in mol-1 cm-1, b is the path length in centimeters and c is the concentration of the compound expressed in moles per liter.4 Spectrophotometry uses Beer’s law to determine the concentration of solutions using absorbance values.5
The particle in a box model is a quantum mechanics exemplar that portrays a particle capable of moving in a small space enclosed by impassable walls. This model can be deciphered analytically with no estimations. Its mathematical uncomplicatedness makes it useful in the calculation of multifaceted physical systems.6 The particle in a box model has immense applications in optoelectronics involving compounds such as conjugated polyene systems.7 Such systems can be represented as a one-dimensional box whose length is the same as the overall bond expanse from one end of the polyene to the other.
Absorbance spectra together with a modification of the particle-in-a-box model can be used to solve for the size of molecules. Most synthetic dyes are made of organic compounds with different functional groups, which result in the formation of conjugated systems. Consequently, the excitation of an electron would result in the movement of the electron from a lower energy level to a higher energy state. In a conjugated system, this movement would be from one end of the chain to the far end of the chain. The “particle-on-a-line” model has been used to describe the movement of electrons along a line.8 This model proposes that the relationship between the energy of an electron and its distance from the nucleus can be explained by the following equation
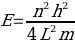
where E represents the energy of the electron, n denotes the state level of the electron, h indicates the Planck’s constant, L represents the length of the line, and m shows the electron mass.9 The movement of an electron from a low to high energy state leads to the absorption of a photon whose magnitude matches the energy change between the two states of transition.10 The wavelength of the absorbed photon can be computed using the following equation
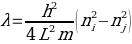
where λ is the wavelength, ni is the lower energy level, and nj is the higher energy level.9 An energy diagram can be used to determine the value of ni and nj by counting the conjugated electron and fill. Moreover, experimental data on the mass of an electron, maximum absorption wavelength, and the Planck’s constant are readily available. Hence, the length of the molecule L can be determined, which will provide information about the size of the dye molecule.
Microtheme
The article describes the creation of a cost-effective scaled down spectrometer that utilizes the inbuilt camera of an Android smartphone to handle images in a spectrometric Android App. The central question was whether it possible to develop an affordable and portable tool to collect spectrometry data. This innovation takes advantage of the computational capacity and the visualization potential of a smartphone for spectrophotometric measurements and evaluation. Additionally, the camera serves as the sensor without the need for an additional processor or communication element. These inventions cut down the cost significantly. The spectrometer, which is attached to the smartphone, encompasses a kit that contains a cuvette holder, an optical fiber, and a white light-emitting diode. Therefore, it is possible to collect spectrometry data in real-time. The calibration of the spectrometer can be done using the Android App. Possible measurements that can be obtained include absorbance, transmittance, and spectral curves. The efficacy of the prototype was validated by determining the concentration of chrome in tanning liquors. The findings were comparable with those obtained from a standardized traditional spectrophotometer. The authors proposed that smartphones could be used to develop cost-efficient spectrometric gadgets for use in science and engineering fields.
References
Ox, J. Color Systems are Categories that Carry Meaning in Visualizations: A Conceptual Metaphor Theory Approach. Electronic Imaging 2016, 1-9.
Ormachea, O.; Villazón, A.; Escalera, R. A. Spectrometer Based on Smartphones and A Low-Cost Kit for Transmittance and Absorbance Measurements in Real-Time. Opt. Pura Apl. 2017, 50, 239-249.
Bormashenko, E.; Shulzinger, E.; Whyman, G.; Bormashenko, Y. Benford’s Law, its Applicability and Breakdown in the IR Spectra of Polymers. Phys. A. 2016, 444, 524-529.
Herzog, B.; Schultheiss, A.; Giesinger, J. On the Validity of Beer–Lambert Law and its Significance for Sunscreens. Photochem. Photobiol. 2018, 94, 384-389.
Gobrecht, A.; Bendoula, R.; Roger, J. M.; Bellon-Maurel, V. Combining Linear Polarization Spectroscopy and the Representative Layer Theory to Measure the Beer–Lambert Law Absorbance of Highly Scattering Materials. Anal. Chim. Acta 2015, 853, 486-494.
Manae, M. A.; Hazra, A. Helping Students Understand the Role of Symmetry in Chemistry Using the Particle-in-a-Box Model. J. Chem. Educ. 2016, 93, 1056-1060.
Muniz, M. N.; Crickmore, C.; Kirsch, J.; Beck, J. P. Upper-Division Chemistry Students’ Navigation and Use of Quantum Chemical Models. Chem. Educ. Res. Pract. 2018, 19, 767-782.
Hatfield, B. Quantum Field Theory of Point Particles and Strings. CRC Press: Boca Raton, 2018.
Thanh Bach’s eportfolio. Web.
Gratzel, M. Heterogenous Photochemical Electron Transfer. CRC Press: Boca Raton, 2017.