This report aims to the benzoine condensation reaction for formation of new carbon compounds. These are cyanide ion and thiamine. Two processes of major significance in the biochemistry field are the sugar synthesis and sugar metabolism processes. Sugar, a carbon compound by itself is the input in these processes, the processes give out a new carbon compound.The recommended practice in sugar synthesis, and metabolism, is that the sugar undergoes a reaction known as benzoine condensation. Notable in this reaction is that it employs the services of two different catalysts that are however observed to function in an exactly similar manner.
Thiamine (C12H17ClN4OS) also known as Vitamin B, is according to Answers.com (2010) a vitamin of the vitamin B complex class that is essential in human diet as its role in carbohydrate metabolism is critical. The website goes on further to point out that intake of the vitamin is a step in ensuring normal neural activity in the body. Beriberi is a decease that is because of the deficiency of thiamine in the body and its symptoms according to Health Grade Inc. (2010) include appetite loss, foot and hand paralysis, heart problems, limb pains, muscle aches, swollen joints and tiredness.
To carry out a benzoine reduction reaction the following apparatus among others will be required a 25 ml conical flask, spatula, Hirsch funnel, watch glass, glass stir rod and an oven. The experiment will also require the following chemicals thiamine hydrochloride, water, ethanol, aqueous sodium hydrochloride solution and benzal dehyde (Chinese Chemical Letters, 2005).
The experimental procedure is as follows; Weigh 0.03g of thiamine hydrochloride and put it in the 25 ml conical flask. Add water to into the conical flask and swirl to make a solution. Ensure that the thiamine hydrochloride dissolves fully. To this solution add 3.0ml of 95% ethanol and swirl the solution until it is homogenous. To this homogenous solution, add 0.90 ml of aqueous sodium hydroxide solution and swirl again until the bright yellow color fades to a pale yellow color. According to Lewis & Waller (1980 p.287), sodium hydroxide is a strong alkali thus used to reduce thiamine hydrochloride. This will approximately take two minutes. Weigh the flask and its contents. Add 0.90 ml of benzaldehyde to the flask and reweigh it to determine a more accurate weight of the benzaldehyde introduced in the flask. Swirl the flask again until the solution in it becomes homogeneous (Mayo, Pike & Trumper, n.d.). Stopper the flask and allow it to stand in a dark place for two days.
After two days crystals will have formed. Initialize crystallization by scratching the inside of the conical flask with a glass-stirring rod (The Journal of Biological Chemistry, 1971). For duration of five minutes, place the conical flask in an ice bath. Using the spatula to break up the crystalline mass that has formed, swirl the flask, and quickly transfer the benzoin to a Hirsch funnel, which has to be situated in a vacuum. Using three portions of ice-cold water wash the crystals. Allow the benzon to dry in the Hirsch funnel. This is done by drawing air through the crystals for 5 minutes. Transfer the benzoin to the watch glass and dry it in the oven, which has o be set at 100˚C (Nagendrappa, 2008). Weigh the benzoin and calculate the percentage yield. Determine the melting point of this crude benzoin (it should be between 129 ˚C and 132˚C). Purify the benzoin by crystallization in from hot 95% ethanol. Cool it in an ice bath and collect the crystals in a Hirsch funnel. Once more, determine the melting point of the purified benzene (it should be between 134 ˚C and 135˚C). Determine the IR spectrum of the benzoin in chloroform.
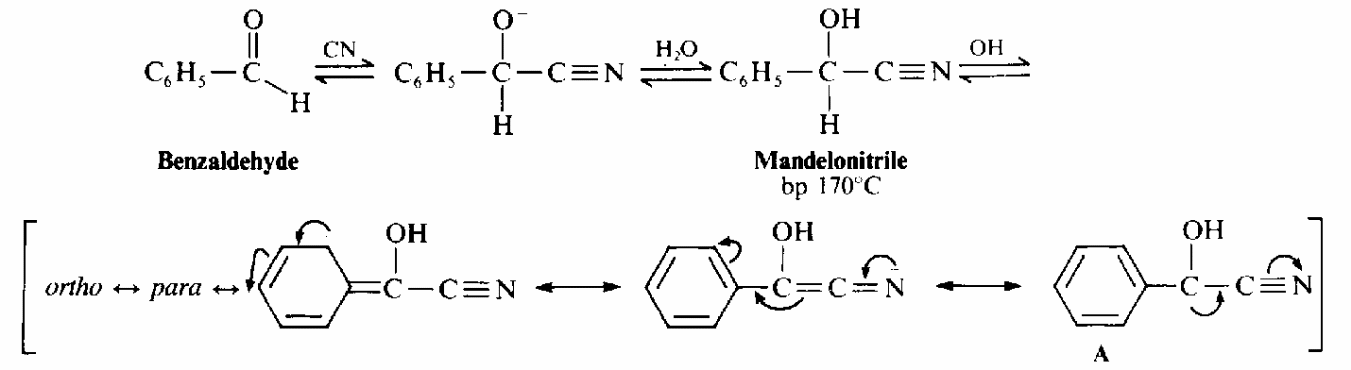
The following is the reaction of the of the process as given by Landis and Nadin (n.d. p. 1)
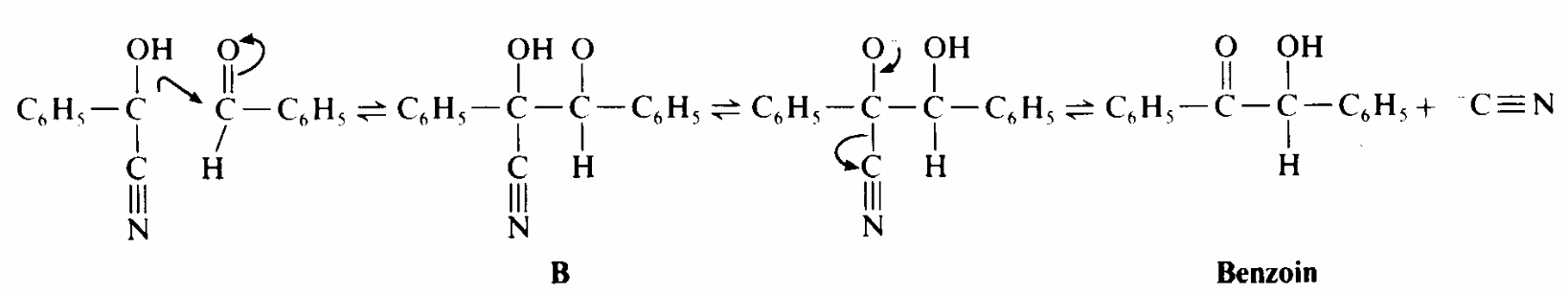
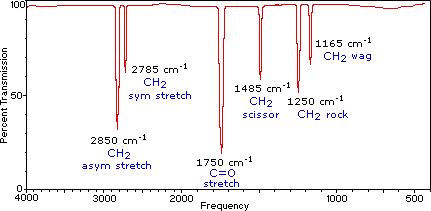
In relation to the IR spectrum of the benzoin, Coates (2000, p.17) observes that high peak frequency band characteristic of aldehydes, associated with the terminal aldehydic C-H stretch. An almond like odor might indicate a nitro compound, benzaldehyde, or a cyano compound , a fish-like smell is often associated with amino or amino compounds, and a putrid or ‘‘bad-cheese’’ odor is associated with certain carboxylic acids. Most enzymes are protein, which are polymers of amino acids. Amino acids provide catalytic functions, serving as acids or bases in one or more steps of the reaction pathway. Enzymes often require coenzymes as cocatyalysts (Wigal, 2000, p. 2).
The conclusion on the experiment is that Benzoine condensation is the reaction between two moles of benzaldehyde and the result of the reaction is the formation of a new carbon compound (Landis and Nadin, n.d., p.1).
References
Anwers.com, 2010. Thiamine. Web.
Chinese Chemical Letters, 2005. Benzoin Condensation in Imidazolium Based Room-Temperature Ionic Liquids. Web.
Coates, J., 2000. Interpretation of Infrared Spectra, A Practical Approach.
Encyclopedia of Analytical Chemistry. John Wiley & Sons Ltd, Chichester. p. 10815–10837. Web.
Health Grade Inc., 2010. Symptoms of beriberi. Web.
The Journal of Biological Chemistry, 1971. Coenzyme Interactions. Web.
Landis J. & Minard R., n.d. Thiamine catalyzed benzoine condensation. 2010. Web.
Lewis, M. & Waller, G., 1980. Thinking chemistry. Oxford: Oxford University Press.
Mayo, Pike & Trumper. n.d. A greener, Biocatalytic Benzoin Synthesis. 2010. Web.
Nagendrappa, G., 2008. Benzoin Condensation. Web.
Wigal T. C. (2000). Thiamine-catalyzed benzoine condensation. Web.