Introduction
The purpose of this experiment is to investigate and estimate the specific heat capacity of two unknown substances. To accomplish this, I used the provided lab scenario to determine the calorimetry of two potential coolants, substances Y and A, and contrasted them with the provided specific heat capacities.
Hypothesis Statement
By determining the specific heats of unknown substances’ chemical reactions, we obtain valuable insights about their thermodynamics and can apply this information to identify them. The heat energy required to increase a compound’s temperature by one degree Celsius is estimated as specific heat capacity (Bardestani et al., 2019). I hypothesize that the unknown substances, Y and A, are expected to be water. On the contrary, the cost of experimentation is a real-world aspect that requires consideration (Pecchi et al., 2020). As a result, the experiment’s cost has been substantially reduced by using a virtual lab as a software simulation.
Methods and Materials
The Materials and Equipment Used
- H2O.
- Substances Y and A.
- Bunsen burner.
- Foam cups.
- A scale.
Methods
- I accessed the virtual lab by loading the lab environment and preparing the experiment’s materials and equipment.
- For experiment 1, I transferred 150 mL of H2O to the beaker and heated it to 100°C by placing the flask on the Bunsen burner.
- Afterward, I determined the mass of 25.0 mL of compound Y using the foam cup and scale and recorded the data.
- I combined the two substances into the foam cup and recorded the water’s final temperature and mass.
- I repeated the steps using 30 mL of water and compound Y and recorded all the data for the second trial.
- For experiment 2, I repeated the steps to obtain data to determine the specific heat capacity of compound A.
Results
In this experiment, an unknown mass of a substance is combined with a known amount of water. The variables in the data table are mc, the mass of an unknown substance; mw, the mass of water; and Tc, the temperature of an unknown substance. Additionally, Tw is the temperature of the water,—qw is the heat lost by water, and Cp,c is the specific heat of an unknown substance.
Sample Calculations
Compound Y (Trial 1)
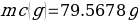
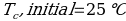
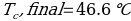
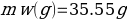
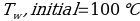
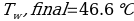
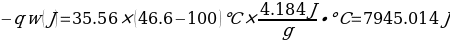
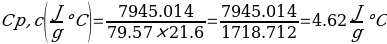
Compound Y (Trial 2)
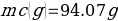
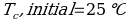
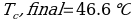
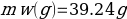
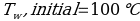
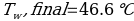
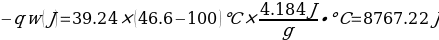
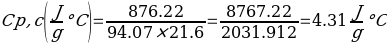
Compound A (Trial 1)
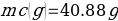
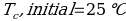
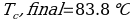
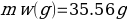
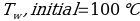
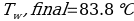
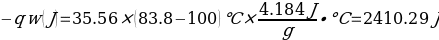
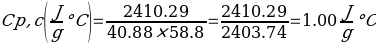
Compound A (Trial 2)
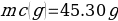
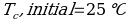
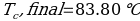
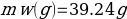
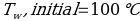
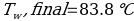
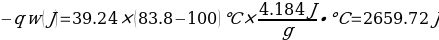
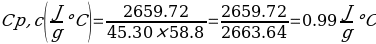
Conclusion
In experiment 1, the two unknown substances were observed to undergo a heat change. This was accomplished by developing an enclosed system (foam cup) (Kisilewicz et al., 2019). Substance Y was water, but substance A was not, so the initial hypothesis was reasonably close to accurate. However, it was determined that substance A was aluminum following the first trial conclusion. The substance Y’s average specific heat capacity of 4.47 J/g°C helps determine that it is probably H2O. The average heat capacity of substance A was 0.995 J/g°C, indicating that the substance is aluminum.
Finally, the average deviation is 0.098 based on the determined specific heat capacity for aluminum, which is 0.897 J/g°C. From this investigation, I learned that calorimetry is a method that can be applied to determine the specific heat produced by a physical or chemical reaction of an unknown substance. Future creations of efficient and sustainable energy technologies, such as hydrogen cells and battery cells, will depend on this concept of specific heat of reaction.
References
Bardestani, R., Patience, G. S., & Kaliaguine, S. (2019). Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. The Canadian Journal of Chemical Engineering, 97(11), 2781-2791. Web.
Kisilewicz, T., Fedorczak-Cisak, M., & Barkanyi, T. (2019). Active thermal insulation as an element limiting heat loss through external walls. Energy and Buildings, 205, 109541. Web.
Pecchi, M., Patuzzi, F., Basso, D., & Baratieri, M. (2020). Enthalpy change during hydrothermal carbonization of biomass: A critical review. Journal of Thermal Analysis and Calorimetry, 141(4), 1251–1262. Web.