The use of instrumental methods of analysis enhances the laboratory capabilities of researchers and directly or indirectly measures important characteristics of substances. This takes on a special meaning when instrumental methods of analysis are used to identify an unknown substance qualitatively. The analysis is unlikely to name the unknown compound under investigation accurately, but it can provide us with several valuable characteristics that can be used for identification. In the present virtual laboratory work, this characteristic was the specific heat capacity, which is a unique thermodynamic measure of a substance that shows the amount of heat required to heat 1 g of that compound by 1 degree (Belford, 2020). So, a significant value of specific heat capacity indicates that a substance is more difficult to heat. To determine the heat capacity of a substance, there is a fundamental equation that relates the amount of heat energy absorbed q, the mass m, and the change in temperature when heating or cooling (Belford, 2020). The purpose of this report is to describe the experiment performed, present the results obtained, and discuss the conclusions.
Hypotheses of the First Experiment
- the coefficient of heat capacity will be higher for coolant Y than for coolant A.
- mixing 25 or 30 ml of water with the same amount of coolant Y has no effect on the final temperature.
- using the virtual calorimetric method, the specific heat capacity of sample Y can be determined.
Hypotheses of the Second Experiment
- the coefficient of heat capacity will be lower for coolant A than for coolant Y.
- mixing 25 or 30 ml of water with the same amount of coolant A has no effect on the final temperature.
- using the virtual calorimetric method, the specific heat capacity of sample A can be determined.
Methods and Materials
Materials
- Distilled water (3 L)
- Foam cup (4 pcs.)
- 250 ml beaker (4 pcs.)
- Scale
- Bunsen burner
- Y-connection
- Compound A
This laboratory work was performed in full compliance with the CHE-121: Principles of Chemistry 1 study guides. The ChemCollective platform was used to conduct the virtual laboratory work. An appropriate library was loaded in the workspace for each coolant, allowing the use of specific materials and samples. The general methodological basis of the work was reduced to mixing some parts of the coolant with hot water in order to determine the temperature difference before and after this mixing. Thus, 25 ml of distilled water preheated to 100°C was added to 25 ml of coolant, and the temperature value after mixing was recorded automatically. The procedure was repeated for 30 mL in order to reduce systematic error, and for another liquid — a total of four similar procedures were performed.
Results
For the present work, the primary results evaluated were the masses and temperatures of the liquids, whether water or coolant samples. Table 1 shows summary data for all four repetitions, for 25 and 30 ml, respectively. The measured mass of the unknown substance, mc, was determined by directly weighing the liquid in the foam cup minus the previously measured mass of the cup itself.
Table 1: Results of Mass and Temperature Measurements in Four Repetitions
In this work, hot water was mixed with a liquid, with the water giving off excess heat. To determine the amount of this waste heat, the standard formula was used:
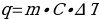
Substituting the mass of water, the temperature change for water before and after mixing, and the reference value for the specific heat capacity of water of 4.184 J/g∙°C yields the corresponding value for the heat energy given by the water to the coolant. In addition, the formula was used to convert from water to liquid:
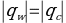
This made it possible to determine the heat capacity of the cooling liquids, assuming that all the heat released by the water was completely absorbed by the sample. Consequently, the average heat capacity values for the two liquids were obtained: 3.767 J/g∙°C for sample Y and 0.989 J/g∙°C for sample A.
Discussion
As part of this laboratory work, two coolants with unknown compositions were investigated. For the purposes of the experiment, these fluids were labeled as Y and A. Identifying the heat capacity of these samples using the calorimetric method will allow their commercial characteristics to be determined. In particular, since both fluids are considered potential company products, it is assumed that a coolant with a lower heat capacity value would be less attractive for sale because it would be heated with less heat transfer (Ramakrishnan, 2021). Thus, it was found that coolant Y had a higher heat capacity value, so in terms of thermodynamic characteristics, it can be considered a more profitable alternative.
It is worth saying that in this experiment, the substances were not qualitatively determined because this was not the task of the study. The calculated values of heat capacities for coolants Y and A can only indirectly suggest which molecules these samples are composed of, but in reality, this is not possible also because coolants can be mixtures and not pure compounds. For example, among cooling fluids, ethylene glycol, water, propylene glycol, and liquid metal alloys are often used in industry (SC Fuels, 2019). Each material is used depending on practical and economic features. It is worth specifying, however, that thermodynamically, water is a more advantageous coolant than sample Y because it has a higher coefficient of heat capacity.
The experiment was able to confirm the correctness of all six stated hypotheses and, most importantly, clearly demonstrated the possibility of calorimetric analysis to determine the heat capacity. In general, the calorimetric method uses the phenomenon of heat exchange with calibrated objects (usually water) to determine the amount of heat and, subsequently, the heat capacity from the energy absorbed or given off (LibreTexts, 2020). Consequently, similar studies can be repeated in actual practice without much trouble. Virtual laboratory work represents simulations of phenomena but cannot be used to obtain real data — which is why extrapolating this simulation is the next step. No problems or weaknesses with the organization were found in this work, so transferring the practice to the real lab should not be problematic.
References
Belford, R. (2020). Heat capacity. Chemistry LibreTexts. Web.
LibreTexts. (2022). Heats of reactions and calorimetry. Chemistry LibreTexts. Web.
Ramakrishnan, B. (2021, June 18). Coolant A has specific heat capacity of 2.200 J/gC and coolant B has specific heat capacity of 3.74j/gC. Which one will serve better as an engine coolant? Why? Quora.Web.
SC Fuels. (2019). A guide to the different types of coolants. SC Fuels. Web.