Goal
To establish antibody purification needs.
Abstract
The variance in the antigen and antibody interaction coupled with the choice of molding these interactions has enabled us to open a new world of antibody use. Today antibody fragments and antibodies are used in immunochemical techniques. It is because of the advanced technologies in the purification methods that they are widely used for diagnostic and therapeutic applications. Today recombinant technology enables limitless combinations of the immunoglobulin fragments, immunoglobulin and tags. This additionally helps in restructuring them tour needs. In this study, the antibody purification techniques are studied with their relevance in the different industries. The processes involved are analyses with the advantages and disadvantages.
Introduction
Downstream processing is the term that is applied to the process of purification which is used for biosynthetic products. The source for the purification could be anything from animal tissue to waste disposal. Downstream processing is essentially inherent in the biopharmaceutical industry for the manufacturing of hormones and vaccines. Insulin is one important example of hormones. Moreover, downstream processing is the purification of all biological products that are required for a certain requirement. These have to be prepared in large quantities for the use of market purposes.
The down streaming has four parts. The first part deals with insoluble removal. Filtration amongst other processes is usually used in this part of the down streaming to segregate components like particulate matter and cells. The second part consists of the isolation of the product. Water being the major impurity is usually removed from the product with the help of ultrafiltration. The third part involves the purification of the product and with the use of chromatography the substances that closely resemble the product in the chemical and physical nature are removed. The last part consists of the polishing of the product. In this the product is made ready to be transported and made stable.
Antibody purification is the term that is applied to the antibodies that have been separated from the antiserum in a mixture of compounds. Once the purification has been achieved the antibodies are utilized in a number of ways. The ways in which the antibodies can be purified are diverse. There are two sources of the antibodies. One is the antibodies from the antiserum in the animals. In this procedure the animal that is chosen is injected time and again with the antigen for the development of the required antibodies. Then its blood sample is taken for the development of the antiserum. This then is further purified to obtain the required antibody. Another method is the cloned cell technique. The cloned cells produce the antibodies from the colony of the identical cells. From this method the antibodies are produced in large numbers and what we term as monoclonal antibodies.
There are many methods available for the purification of the antibodies. The method that is chosen is largely dependent upon the class/subclass to which the antibody belongs, to what use it will process for, and the species in which it is raised. The antibodies that are developed from the cultures of ascites, serum or tissue undergo a number of methods like gel filtration, ammonium sulphate precipitation, protein A/G purification, Immuno affinity purification and ion exchange chromatography. This results in a more purified form of the product desired.(Boi, 2007).
The technique used for the purification of the antibodies depends upon the purified medium. For example the antibodies that are made from eggs are treated altogether differently than the antibodies that are produced from the antiserum of an animal like rabbit. Ideally the purification of the antibody is solely dependent upon the substance that binds to the antibody and keeps it in place while the rest of the unwanted molecules are wasted away. The quantity of the antibodies that are generated depends upon the need. For a research there may be not be a mass requirement of the antibody production but it is vice versa when it comes to therapeutic purposes and clinical trials. Also the manufacturers are interested in the mass production.
The monoclonal antibodies are very important as they are sought for the treatment of the cancer. This is because of the fact heat they have a tendency to be attached to the radioactive agents and also other compounds. This in such a state can then be introduced inside the body. These then have the ability to only target the cancer cells in the body.
Monoclonal antibodies (mAbs) are soluble stable structures that are unique in that they provide the platform by which other products can be processed when one mAb is already processed. They possess common process flow sheets and hence can be manufactured in many facilities in large amounts and have very little process time. For these reasons they are also cost effective as well as provide flexibility in the options available for the processing (Costa & Cabral, 1991).
Today with the varied therapeutic uses, the purified forms of the antibiotics have become increasingly important. The antibody purification systems are also important as there is much need of the purified antibodies in the research as well. The manufacturers are always on the lookout for new processes that are cost effective stable and easier to use. For this newer mechanisms have been developed and there have been advances made in the membrane technologies which would be detailed later in the study. The chromatographic separations have been successful over time which have been enhanced by the modified ultra filtration processes and the structure of the membranes used.
The disposability options in the membranes have given rise to new opportunities which has enabled to cut costs as well as save time. Such measures have brought more diversified and purified forms of antibodies that have been used in the research to produce potent vaccines and cures for diseases like cancer (Mulder, 1996).
In this case study the downstream processing of the monoclonal antibodies is analyzed with special reference to the Chromatographic and membrane separation s well as ultra-filtration and di- filtration. The different processes that are used in the purification of the antibodies are compared and contrasted in the Literature review with the advantages and disadvantages of using the different mechanisms. The processes are analyzed from an unbiased viewpoint and the findings are recorded. The conclusions are drawn from the research material collected.
The research material has been collected form authenticated sources which include books, journals by authors whose works have been established in the field. The literature collected is unbiased and in a step wise manner clearly identifies the different procedures from the starting history till the latest development and modifications of the different processes involved in the antibody purification.
Background/ literature review
Purification, separation and concentration of the different products are a problem that is faced by the biopharmaceutical industry. The scientific development in the past 200 years has resulted in many breakthroughs in the separating processes. Most recent are the conventional separating processes. Here semi permeable membranes are utilized for the separation. The membranes and the membrane processes were initially utilized as analytical tools in the biomedical laboratories. Soon they were incorporated in the industrial sector (Ghosh, 2002).
Membrane processes work very differently. The different components are segregated through physical means at normal temperature. The components are not damaged and are not chemically altered. This method is ideal for large scale operations and also for the batch type processes. The membrane selection depends upon the degree of separation required, constituents and the volume of the solution (Zeng & Ruckenstein, 1999).
In 1980’s and early 1990’s the processes that were used for the mAb purification were state of the art in comparison of what we have today when there is formulated process knowledge available and there are many separation media choices available. Some of the earliest processes that were used were the depth filtration, microfiltration, size exclusion chromatography, four or more chromatography steps, affinity chromatography with protein G in addition to the protein A, challenging seperative method for large scale production, conventional capture columns to protect protein A. Earlier the downstream processing was achieved in cold. For the production in kilogram scale the bioreactors were used that were of very large size. Volumetric productivity was increased to capture resin selection; this was done in order to handle the large volumes of feed quickly in contrast to the handling in batches (Shire, 2009) (Mulder, 1996).
History
Chromatography started somewhere in the middle of the 19th Century. Chromatography is the term that is taken from the words “color writing” and was first employed for the segregation of the chlorophyll which is a plant pigment in the beginning of the 20th Century. It was by 1930- 1940’s that newer techniques evolved for the separation processes. It was in late 1940’s- 1950’s that triggered the chromatographic techniques by the works of Richard Laurence Millington Synge and Archer John Porter Martin on the subject. It was the backbone of the work of these men that the different types of the chromatographic techniques evolved. Of these were the gas chromatography also later known as high performance liquid chromatography and paper chromatography
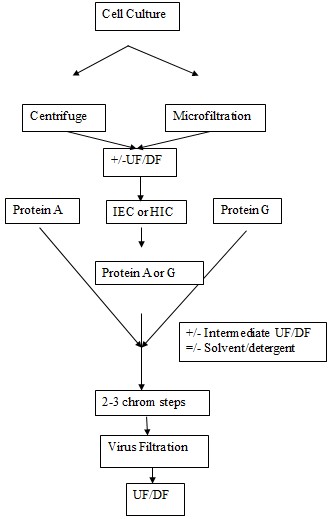
Current approaches
Today many of the companies have simplified the processes by defining a platform of purification. This is constructed on the common sequence of the unit operations principle. One such common platform is shown in the following figure.
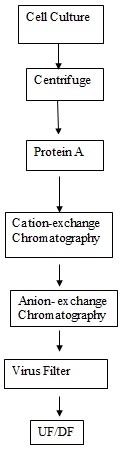
The mixture is first of all put through centrifugation for the clarification. The n it is subjected to filtration. The protein A chromatography
For the treatment of acute infections there are antibody fragments like single chain fragment variable (scFv) and fragment antigen binding (Fab) are used. Example of the Fab is the ReoPro which is used for the treatment of the cardiovascular disease, DigiFab for Digoxin overdose and CroFab for the sanke venom. The fragments are also used a a help in diagnostic tools. Example is arcitumomab for the imaging of the malignancies in the gastrointestinal area (Gottschalk, 2009) (Nunes & Peinemann, 2001).
Terms used in Chromatography
- Analyte : The removed substance in chromatography is termed as analyte.
- Analytical Chromatography: This is the term that is used when the quantity of the analyte in the substance has to be determined.
- Bonded Phase: The stationary phase, which is covalently bonded to the column tubing or the support of the particles is termed as bonded phase.
- Chromatogram: The output of the chromatography that is obtained in visual manner is termed chromatogram. The peaks of the chromatogram predicts the different concentrations of the components in the mixture
- Chromatograph: this is the equipment that is used in the chromatography. Example: liquid or gas chromatography.
- Elute: The mobile phase that leaves the column is termed as elute
- Eluent: This is the solvent that is responsible for the carriage of the analyte.
Different techniques of chromatography
Colum chromatography
This is termed as column chromatography because the stationary bed is located inside the column. The particles that are retained inside the length of the column are of support coated with the liquid stationary phase or the stationary phase and this is termed as the packed volume. These can also be dispersed alongside the wall of the column leaving enough space for the mobile phase in the middle of the tube which is termed as the open tubular column. The rate of movement and the difference of the movement are calculated according to the retention time.
Modified version of the column chromatography is the flash chromatography. The only difference is that the solvent is introduced into the column with the application of the pressure. Hence the separations occur in a speedier manner. The separations that are attained in this manner are far superior to those attained by the older version of the column chromatography. Fraction collectors and distillers are used to make the system more automated and saving on to cost and time. The use of gradient pumps with these systems helps in the less utilization of the solvent and the separations are done at a faster pace (Nunes & Peinemann, 2001).
Planar Chromatography
In this the stationary phase is on a plane hence it is termed as planar chromatography. The plane could be the paper on the stationary bed which would be the paper chromatography or it could be the solid particle layers spread over glass plate, which is termed as the thin layer chromatography.
Paper chromatography
In this technique the chromatography paper is used. The paper is taken with the application of a little application of the sample solution. The paper is then placed in a layer of the solvent which is then placed in the jar and is then closed so that no air can pass. The solvent then starts to gradually rise up the paper making the mixture to rise with it. As the paper is polar and made of cellulose material the components in the mixture that are not polar will result in travelling the farthest. The polar substance will bond significantly more strongly with the appearance with the result of not travelling much farther.
Thin layer Chromatography
This is more in line with the paper chromatography. Here for the stationary phase the gel or silica is used as a thin layer. It is far superior to the paper chromatography as the separations are not only obtained in a more rapid manner but are also of a more superior quality (Aldington & Bonnerjea, 2006).
Displacement Chromatography
A high affinity molecule called displacer is utilized in this technique.. It works by competing with the affinity sites. The molecules that have lesser affinity will not be allowed to attach to the sites. These molecules would be displaced. This type of chromatography is far superior to the elution chromatography as the as the components do not formulate the peaks as is the case in the eluting chromatography. The nonlinearity of the isotherms provides for a greater feed to be separated and the components that are recovered are of a much larger quantity.
Gas chromatography
This is also termed as gas liquid chromatography (GLC). The mobile phase in this type of chromatography is the gas. This type of method involves the use of capillary or column and it is always packed. The principle behind the working of this type of method is the partition equilibrium. This equilibrium is between the mobile gas like Helium and the solid stationary phase which is composed of the liquid silicon. The inside of the capillary column is responsible for the residence of the stationary phase. This method is not ideal for the separation of the proteins as it involves very large amounts of heat (Tennikova et al, 1990).
Liquid Chromatography
Here the mobile phase is the liquid. This type of chromatography can be carried out both in plane as well as column. The high performance liquid chromatography (HPLC) means the particles are packaged to a very small size under very high pressure. The column consists of the stationary phase and is in the packed state. The sample is introduced in the column at a very high pressure. According to the polarity of the mobile and stationary phase, HPLC is divided into two parts. Normal phase liquid chromatography (NPLC) is the term that is used when the stationary phase is more polar that its counterpart which is the mobile phase. Reversed phase liquid chromatography (RPLC) means that the polarity is more for the mobile phase is more polar as compared to the stationary phase. This phase has more use (Costa & Cabral, 1991).
Affinity chromatography
The non covalent bonding of the molecule and the analyte is termed as the affinity chromatography. The proteins that are bound to tags are used in this purification process (Thoemmes & Kula, 2008).
Ion Exchange chromatography
In this type the ion exchange method is used for the segregation purposes. This type of chromatography is used in both the planar mode as well as in columns. Charged stationary phase is used to separate the charged compounds like proteins, peptides and the amino acids. This charged ion which is exchanged resin then interacts with the oppositely charged ions and are subsequently removed (Tennikova et al, 1990).
Size Exclusion chromatography
It is also commonly known as the gel filtration chromatography and gel permeation chromatography. In this the particles are segregated on the basis of their size. The smaller particles get trapped inside the mobile phase and hence are eliminated. This technique is a low resolution technique (Ghosh, 2002).
Downstream processing
In the downstream processing it very inherent that the biological activity and the integrity of the protein is retained and at the same time removing all the impurities and the additives in the mixture. For the attainment of this fact it is important to be knowledgeable about the products stability and impurity. This is essential as the process of r the purification would be set accordingly to attain the best possible result for the use in the clinical trials. Product impurities during its manufacturing should be looked at and consequently removed.
The downstream processing of the recombinant antibodies is divided into four parts.
- Initial isolation of the host cell from the soluble product
- Stabilization and the concentration of the protein for removal of impurities
- Removal of residual host impurities
- Single or multiple polishing step application is used in order to remove the impurities, ensuring clinical suitability and developing effective, economical process.
The affinity chromatography has limitations when it comes to the antibody fragment capturization. This is due to the high cost of the cleaning reagents and the resins. The low affinity of the resins requires large bed volumes. This means that repeat column cycling is also required (Thoemmes & Kula, 2008). This in turn requires a lot of batch cycle time. Then again column cleaning would be required and sometimes with the low affinity resins it becomes inherent to use expensive cleaning solutions.
Chromatography
The process of segregating the components of the dissolved components in mobile phase in accordance to the transport rate of the components in the stationary phase is termed as chromatography. Simply put it is a set of procedure that are usually undertaken in the laboratory for separating the mixtures. The transport rate of the components is directly proportional to the relative affinity. In this process the components pass from mobile to stationary phase through a process which is termed as sorption. The release from the stationary phase is known as desorption. The lesser the affinity of the molecule, lesser time would be spent in the column. The components are segregated as they travel through the stationary phase (Kelly, 2007).
Chromatography can either be analytical or preparative. Analytical chromatography involves dealing with the little amount of the mixtures and the preperative is the separation of some components of a mixture that need to be used later.
This method is employed by the biopharmaceutical companies to segregate and purify the different products. There are many different chromatographic processes. The different chromatographic mechanisms are based upon the mass transfer and the segregation of the two phases that is the stationary and the mobile phase. The different chromatographic techniques are affinity chromatography, ion exchange chromatography, gel permeation chromatography and liquid solid chromatography. These different techniques are based on the mass, strength, side chains, ionic and polarity (Boi, 2007).
The chromatographic process is dependent upon the surface tension between the eluent and the solute. In the stationary phase the solute with the strong interaction moves slowly in respect to the eluent flow. The solute with an affinity for the eluent molecule moves at the same speed as the eluent molecule in the column. (Osada, 1992).
Downstream processing: Chromatography
Chromatographic and membrane separation
While using the basic chromatography a column of steel is used this is packed with resin. This is the stationary phase. It is composed of porous beads which are synthetic, mineral or polysaccharide medium and is conjoint to unique functional groups that utilize variant seperative principles. Different components in the feed are introduced in through the raisin. The components get segregated as they have variant affinity to the functional groups. The separation in the resin is either flow through or retention in which the target is selected and kept and contaminants are wasted. The opposite of this is the flow through process. Hence the column chromatography forms the basis in the of the bio separation process but for this reusable large columns are always needed for the segregation purposes (Thommes & Kula, 2008).
For the purpose of flow through also referred to as polishing the membrane based concept is more economical as they are disposable and cost less. The method involves the use of oversized columns in order to balance the throughput. This has a direct bearing upon the infrastructure, costs and design as the buffer volume and space need to be in accordance to the procedure. The membrane chromatography comes useful as the membrane is thin and porous. It consists of a synthetic multilayered cartridge. Another feature of the membranes is that they functional groups which are equal to the representative resins. Another benefit of using membrane chromatography is that unlike the column there is no need for the checking, cleaning , re fuelling , packing or maintainace (Dutton, &Scharer, 2006).
The advantages of the membrane adsorbers can be fully illustrated through the anion exchange chromatography. DNA and viruses are not easily mixed with the pores of the basic resins. This process requires low efficiency and mass transfer resistance.
This causes lower efficiency and transfer resistance. The polishing steps in column chromatography demand very large sized columns whereas in the membrane chromatography the membrane adsorbers have binding sites which have specific affinity for the specific solutes. This process takes place through convection with minute pore diffusion. Even the process time is decreased as much100 fold with shortened buffer consumption. The reason is the hydrodynamic benefits which facilitates the membrane adsorber with increase in the flow rate as compared to the column chromatography.
DNA can be separated from the protein with the help of the anion exchange membrane. For this a membrane bed of 4mm in height and with a flow rate of 600cm/h is used. This provides the ideal environment for larger molecules like DNA and other viruses to be attached to the binding sites at the membrane pores (Li, 2008) (Drioli & Giorno, 2009).
The most important aspects in the calculation of the costs of unit operations are disposability and the capacity. It is seen that the membrane devices have a far greater throughput. But when volume is taken as base for the comparison between the membranes and resins then the membranes are seen to be more expensive than resins. Yet again the membranes take the lead as this factor is negated by the smaller size of the membrane devices which is responsible for the reduced time of buffer and consequently reducing the process time altogether. For the cost analysis a cost model is utilized which is able to separate the indirect costs as well as the direct costs involved. The categories covered by such a model are the labor, consumable equipment, media, consumable chemicals, materials and capital equipment (Costa & Cabral, 1991).
The cost factors associated with the column chromatography are the costs of the buffer preparation, consumption and the equipment costs. For the membrane chromatography the costs are for the consumable equipment. Moreover in column chromatography the use of buffers and resins is directly proportional to the production scale hence it is clear the disposable mechanism is more economical. When the loading capacity is low then the column are more an economical choice. For example at 10kg/L capacity the columns cost five times the price of the other disposable mechanisms.
Membrane Separation process
Membrane separation is dependent upon the difference in the transport rate of the different constituents in the mixture. This transport rate is a measure of the driving force of the components, their concentration and mobility. The mobility is directly proportional to the physical attributes and the size of the molecules while the concentration is dependent upon the permeating component compatibility (Walker & Rapley, 2009).
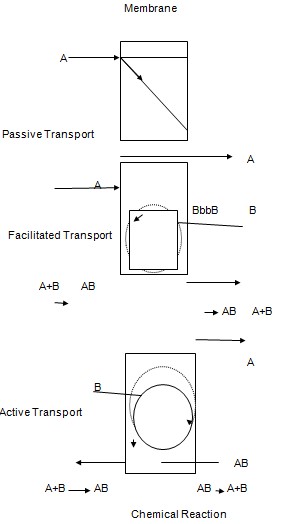
- Passive Transport: In this the components are separated through the driving force which are the electrical potential differences, concentration and the hydrostatic pressure. The hydrostatic pressure difference results in the volume flux. The chemical components re separated with the different hydrodynamic permeability of the membrane. The concentration difference results in the separation of the components according to heir different diffusivities. The electrical potential difference results in the separation of the chemical components according to the different motilities of the molecules (Wofsy & Burr, 1969).
Types of Membrane separation processes
Membrane separation processes are different according to the different hydrostatic pressure applied. It is also dependent upon the economic relevance, technicality and the area of application. The hydrostatic pressure gradient involves the use of ultra-filtration, microfiltration, gas separation or reverse osmosis. In the gas separation membrane distillation and prevaporation can also be used to separate the molecules (Baker, 2004).
Pressure Driven membrane process
This includes ultra-filtration, microfiltration, gas separation and the reverse osmosis. The mechanism is explained in the following diagram.
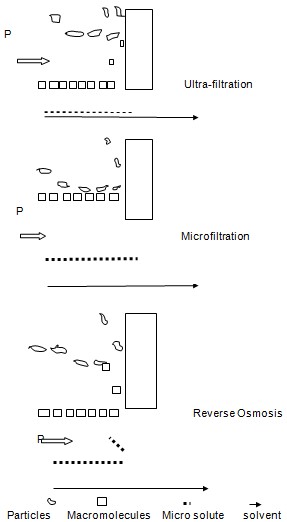
Ultra-filtration differs from the other processes due to the nature of the type of the membrane and the size of the particles that have to be separated. The components that have to be separated are brought to the semi permeable membrane surface. The particles are separated according to their sizes in a sieving fashion. The process itself is named as ultra-filtration when the particles that are separated do not exceed the size of 0.1µm in diameter. In ultra-filtration the hydrostatic pressure usually ranges from 2 to 10 bars. The membrane that is used in the ultra-filtration is asymmetrically made with the pores ranging from 1- 20 nm in diameter (Gottschalk 2009).
Membrane chromatography
Out of all the methods the membrane chromatography is seen to be the most promising. This is because of the fact that it is easier and much more cost friendly for the mass production. Moreover it is very easy to scale up and set up. The structure of the membrane chromatography is three dimensional hence it is more apt in the purifying of the proteins. The large dimensions of the pores make more active sites present for the binding of the large molecules like the DNA, viruses and plasmids. The capacity naturally increases with the size of the pores and hence the flow rate is increased. This is 100 times to that of the resin bead chromatography (Drioli & Giorno, 2009).
The membrane chromatography has two formats. Membranes with the disposable capsules and cartridges have high adsorption rate, high resolving capabilities and high flow rates. Membranes of 80 layers are stacked together with the stainless steel housing, which makes it resilient against high pressure. It has high capacity and resolving power and is also reusable.
In the membrane chromatography there is covalent linkage between the functional groups and the porous polymer ultra filtration membrane. These systems provide wide flow rates, high resolution and stable binding. Their unit operation set up is very little because of the ready to use storage properties.
New Breakthroughs in the membrane technology
To be more competitive and have enhanced results there has been newer development in the membrane chromatography. This is in the form of the hydrogel composites. These structures are responsible for the creation of high capacity macro porous membrane and hydrophilic products and so help in the increase in the binding capacity. The membranes these days are being chemically modified for the improvement in the binding sites, stability and throughput. They are focused in maximizing the yields and reduce fouling.
Resin Bead chromatography
There are many limitations of the column chromatography. The flow rate of the purification is directly dependent upon the diffusion in the resin bead’s pores. The resion’s ion exchanges beads are present inside the internal bead pores. This makes the travel path of the biomolecules longer and caused impediment. As the accessible area becomes restricted, it becomes unable for the beads to purify the large molecules. The large biomolecules that are left un attended in this manner are the plasmids, viral vectors, vaccine components and blood factors. The method provides very limited capacity as the beads bind only to the outer surface which reduces the availability of the binding sites.
Moreover the hardware becomes unreliable and expensive as the height of the resin bead in the column requires time for the diffusion into the bead pores. The virus and DNA column capture is not sized by capacity rather by the flow rate. So when very large batch processing is required the columns sizes are extravagantly bigger than the requirement of the capacity. This causes the resin bead chromatography to become restricted and unreliable (Pinnau & Freeman, 2000).
Comparison between the resin based and membrane chromatography
The diffusional limitations in the resin based chromatography make proportional flow rate maintenance in the scale up to be expensive and difficult. The scale up in the membrane chromatography is not like the resin based. Unlike the columns the scale up is linear procedure. Capacity is directly proportional to the volume. This means that it is directly proportional to the membrane layers, membrane unit and the bed height.
The dynamic binding capacity of the membrane capacity is far more because of the fact that the thickness of the membrane bed is not important but it is otherwise in the resin based chromatography. Hence the flatter beds of the membrane chromatography provide additional scaling up.
Flow is directly proportional to the pressure uniformity. High pressure in the resin based chromatography leads to the compaction of the bed. This results in the poor separation resolution because of the decreased flow rate.
Limitations of column chromatography
Major limitation of the procedure is the irregular flow inside the column chromatography. The variant absorption and desorption is caused by the variance of the flow rates inside the columns. Sometimes the binding sites inside the columns are free and a part of the column gets packed on the other hand. This breakthrough and the product bondage occur at the same time, causes destruction of the column resolving power. Fluid channeling is often the result of poor packed columns. This causes impediment of the separation resolution. The separation of the target substance is entirely dependent upon the separation resolution and a failure of it means the failure of the product recovery.
Ion exchange chromatography over column chromatography
The diffusion time in the ion exchange chromatography is decreased and this causes a very speedy purification. The structure of the membranes involved has a direct bearing on the end result that is the purification of the product desired. A membrane that is named as the polyether- sulfone membrane is recently developed which has very minute absorption rate for the non specific protein absorption. This is an additive benefit for this property is very beneficial for the chromatographic procedure.
Ultra-filtration and di-filtration
Anisotropic semi permeable membrane is used in the Ultrafiltration process. The solvents and the macromolecular species are segregated according to the shape of the molecules. The ultra filtration is used in the biological species to increase the solute concentration. In the solute purification and desalting the solution is purified from the low molecular weight substances like non aqueous solvents and salts. To the ultra filtered solution the solvent is mixed for the removal of the micro solutes. The filtration rate is used for the process and this whole procedure together is named as dia filtration.
The ultra-filtration membranes are employed to enhance the selective product. This is achieved with the mechanism retention of molecules that exceed the size of the pores of the membrane. This method is also cost effective. The samples that are used are segregated according to the size. This method is especially good with the downstream processing which requires purification. The molecules that need to be separated are segregated from other non aqueous solvents, low molecule weight substances and salts. The di-filitration is the process where the micro solutes are removed when solvent is added to the solution for ultra-filtration which is equivalent to the speed of filtration.
The large sized solutes like suspended solids, proteins, suspended solids, bacteria, oil are segregated through a low pressure mechanism and the alkaline compounds, water and acids pass through. Ultra-filtration is very important as fermentation and harvesting processes depend on it. Also is the dependency of the macromolecular nature of the products that are used in the bioengineering. The ultra-filtration membranes separate the product and the enzymes with the unspent substrates in continuous and batch reactor. The ultra-filtration in optimum environment has the ability to segregate enzymes without the loss of the enzyme activity. Through the ultra-filtration process the substrate is gradually passed into the enzyme reactor. The resultant product is either directly passed through or is kept behind. The product’s molecular weight distribution is dependent upon the absolute ultra-filtration pressure, minimal molecular weight cut off, and residing time of substrate, temperature and substrate to enzyme ratio.
In biopharmaceutical manufacturing peptones are commonly used for the enhancement of the cellular growth. The problem with hydrolysates like peptones is that they get contaminated by the endotoxins and hence the elimination of the endotoxin is very important in this field. Ultra-filtration comes handy as it easily removes the hydrolysates. Most of the vaccines for children and infants to combat diseases like invasive pneumonia and meningitis are easily developed because of the ultra-filtration process. It streamlines an approximate of 30 purification steps for the removal of impurities in an effective and timely manner. Hollow fibre ultra-filtration is used for the removal of the contaminated proteins in the hepatitis B surface Antigen (HBsAg) broth. The Acrylate ultra-filtration membrane is used to counter the foot and mouth disease virus broth.
The isolation of the proteins from the buffer parts is obtained through the process of ultra-filtration in exchange for the concentration, desalting and the buffer. The method is good in the removal of sugars. This is done in exchange of PH change, difference of ionic environment; free ligand separation from protein bound ones, non aqueous solvents and low molecular weight components. The advantage of the ultra-filtration is that in this process there is no need to change the phase at room temperature and the purification, desalting and concentration is achieved through one single step. This is contrary to other methods available. The cellulose acetate ultra-filtration membranes that have low capacity for protein binding are employed for the revival of Papain. Example is the use of Pectinase enzyme for fruit juice clarification process. With the use of ultra-filtration the fruit juices rae clarified without having to put them under other harsh methods like the pH de-naturation and heat. Another advantage of these membranes is that they do not degrade even after prolonged use. They require little maintenance and last without ball washing and the chemical washing from time to time. The method is twice used for the whey and milk processing. The proteins of the whey are removed through the ultra-filtration process. The permeate is subjected to lactase which helps in the splitting of the lactose into the galactose and glucose. In this process second ultra-filtration is used for the enzyme recovery from the substrate (Boi,2007).
Microsolutes: Antibiotics and Vitamin separation
Many of the Antibiotics like erythromycin, penicillin V,benzylpenicillin and clavulanic acid are separated from the microbial colonies and the high molecular components through the process of ultra-filtration. This method in conjunction with the difiltration is more effective than the filter aids, flocculants and rotary vacuum filters. The vitamins and hormones pass through these membranes and this way of filtration and concentration is a much better method than other for the preservation of the antibiotics and Vitamins.
Ultra-filtration is also used for the cheese processing. Ultra-filtration increases the milk protein use in the recovery for the product stage of the whey formation. There are two stages of the ultrafiltration in the process of whey formation.
In the first stage whey is separated from the salts and lactose. The second stage involves the working of the membrane in conjunction with the reverse osmosis to concentrate and segregate the lactose. This establishes the fact that ultrafiltration is used for many important purification processes and because of the process quality and stability it is used to purify so many varied products. The ultrafiltration is also used in the membrane chromatography sometimes in conjunction with the dia filtration. This produces the required purified forms of the antibodies.
Effluent treatment and Portable water
Hollow fiber ultra-filtration membrane effectively removes the microorganisms. They have the ability t retain the virus and the bacteria. This is done in the recirculation or even single pass mode. At the industry level ultra-filtration membrane bioreactor is utilized for treating industrial effluents. This type of ultra-filtration is advantageous as compared to the other treatments for waste water as it meets the regulations for the discharge. The space required for the plant is less and also the cost of the clarifiers used is low hence it is also cost effective.
Findings
There are many methods available for the separation purposes. The antibody purification is done by a few methods in a favorable manner while some methods which for example have very high heat are not good as they tend to degrade the protein molecules. Of the methods the membrane separation has been seen to favor the antibody purification through which the molecules that are required are segregated by the ultra-filtration method and the di- filtration.
The more advanced and newer membrane chromatography methods provide much more binding capacities and the resultant increase in the flow rate of the endotoxins, DNA and viruses separation now provide the biopharmaceutical manufacturers more opportunities than before.
The membrane chromatography has provided the options of the disposable membranes. Moreover the structures of the membranes have been modified to provide them with additional stability with the additional binding sites as the membrane structures are chemically modified. Chromatography has been carried out since years but the processes which involve the use of membrane have been found to be additionally effective as far as the purification of the antibodies is concerned. This is because of the fact that the bigger molecules find more binding sites on these membranes; such molecules are the DNA, endotoxins and the viruses. This makes the antibodies that are required to be in a more purified form. Also the size of the mechanism is suitable according to the capacity of the procedure. The option of the membrane chromatography has been found to be more cost effective and reliable.
There are many therapeutic uses of the separated and purified antibodies and there is a very big market for these purified antibodies. The study establishes that the use of these purified forms of the antibodies not only is essential for the clinical use but many fruitful researches are also carried out through the attainment of the purified forms of these antibodies.
Today with the advancement of the technologies the vaccines and therapies are becoming abundant throughout the world. The membrane chromatography has opened doors to a world of opportunities in the streamlining of production. Another very important aspect that the manufacturers benefit with is the decrease in the cost.
Conclusion
Disposable equipment nowadays is favored and universally accepted as the best means in the biopharmaceutical industry. It is a very favorable investment for the hardware especially for limited use. The membrane chromatography proves this by being economically when it comes to separation of impurities. Such material greatly reduces even the labor costs which are otherwise incurred for the cleaning and support.
The genetic therapies as well as protein based therapies both require the manufacture of the purified forms of plasmid preparations as well as proteins. These proteins need to be totally free of the contaminants like the endotoxins, host cell proteins, nucleic acids, enzymes and the viruses. For the purpose of purification of the proteins it becomes extremely crucial to develop processes that are cost effective and save on to time. The traditional method of the chromatography has been seen to have many drawbacks as per the findings of the study. This is especially true with the very large molecules, with the slow diffusional rates and weight of the molecules. The technology that has answered the dilemma till now is the membrane chromatography. It is the answer to the purification as it has the ability to speed up the cycle times and this enables more therapies to be produced in the market by the manufacturers.
The membrane chromatography has the capacity of handling high flow rates and can easily purify the endotoxins, viruses and the DNA molecules. This is good news to the biopharmaceutical manufacturers. The membranes are resistant to the volumetric flow rates. The smaller size of the membrane units also makes them much easier to handle as compared to the columns.
References
Aldington, S. & Bonnerjea, J. (2006) ‘Scale-up of monoclonal antibody purification process’, Journal of chromatography, 848(1), 64-78.
Baker, R.W. (2004) Membrane technology and applications. USA, John wiley and sons.
Boi, C. (2007) ‘Membrane adsorbers as purification tools for monocolonal antibody purification’, Journal of chromatography, 848(1), 19-27.
Costa, C. A. & Cabral, J. S. (1991) Chromatographic and membrane processes in biotechnology. USA, springer.
Drioli, E. & Giorno, L. (2009) Membrane operations; Innovative separations and transformations. Germany, Wiley-VCH.
Dutton, R.L. & Scharer, J.M. (2006) Advanced technologies in biopharmaceutical technologies. USA, Wiley Blackwell.
Ghosh, R. (2002) ’Protein separation using membrane chromatography: oppurtunities and challenges’, Journal of chromatography A, .952(5), 13- 27.
Gottschalk, U. (2009) Process scale purification of antibodies. USA, Wiley- Interscience.
Kelly, B. (2007) ‘Very large scale Monoclonal Antibody Purification: The case for conventional unit operations’, Biotechnology Progress, 23(1), 995-1008.
Li, N.N. (2008) Advanced membrane technology and applications. Canada, Wiley- Interscience.
Mulder, M. (1996) Basic principles of membrane technology. USA, Springer.
Nunes, S.P. & Peinemann K.V. (2001) Membrane technology in the chemical industry. Germany, Wiley-VCH.
Osada, Y. (1992) Membrane science and technology. USA, M. Dekker.
Pinnau, I. & Freeman, B.D. (2000) Membrane formation and modification. USA, American chemical society.
Shire, S. (2009) Current trends in Monoclonal antibody development and manufacturing. USA, Springer.
Tennikova, T.B., Svec, F., & Belenkii, B.G. (1990) ‘High-Performance Membrane Chromatography. A Novel Method of Protein Separation’, Journal of chromatography, 13(1), 63-70.
Thoemmes, J. & Kula, M.R. (2008) ’Membrane chromatography- An intergrative concept in the downstream processing of proteins’, Biotechnology Progress, 11(4), 357-367.
Walker, J.M. & Rapley, R. (2009) Molecular biology and biotechnology. UK, Royal society of chemistry.
Wofsy, L. & Burr, B. (1969) ‘The use of affinity chromatography for the specific purification of antibodies and antigen’, The journal of Immunology, 108(2), 380-382.
Zeng, X. & Ruckenstein, E. (1999) ‘Membrane chromatography: Preperation and application to protein separation’, Biotechnology progress, 15(6), 1003- 1009.