Introduction
Human health is a sensitive state of complete physical and psychological well-being observed in the absence of cognitive and somatic diseases. Health as a whole should be viewed as a function of the many variables that determine a person’s final state: this includes biological parameters, mental functions, and the ability to respond adequately to the external environment. For example, if a person is affected by a viral infection or has cognitive abnormalities, his or her health is said to be bruised. Recently, researchers have become increasingly convinced of the phenomenon of microbiological predictors of health related to the gut. The idea of the influence of the gut microbiome on body health is driven by systematic refinement of medical, biochemical, and environmental studies of the human gastrointestinal tract and the search for direct links with different states of human health. It is noteworthy that until recently, scientific articles often used the term “gut microflora” to mean the totality of living microorganisms that indirectly or directly affect the body. The move to use the term “microbiomes” is not accidental: the gut is no longer viewed as just a collection of bacteria but has come to be perceived as an ecosystem unique to each individual, combining unique biological, chemical and physical factors, resulting in an exclusive body microbiome.
The focus on the patient’s digestive system was not accidental because it is in the intestine that the largest number of internal human microorganisms live. Most of them are symbionts that help perform essential organismal functions: for example, Gram-negative E. coli inhibits the development of pathogenic bacteria and helps synthesize vitamin K (Zhang et al., 2021). Any imbalance in the number of symbiotic and pathogenic bacteria and a deficit or surplus of gut symbionts leads to disease. Thus, it is believed that one of the most important immune functions is to protect the gut from colonization by pathogenic bacteria, which affects human health in the long run. In reality, however, the role of the gut in shaping a healthy body is much broader. The gut microbiome is thought to shape human immunity through the synthesis of short-chain fatty acids, which in turn are involved in the differentiation of immune regulatory T-cells (Ruohtula et al., 2019). Thus, T cells can control the strength and duration of the immune response through the regulation, hence the name, of t-helper cells, which allows the formation of the body’s autoimmune defense, among other things. In addition, symbiotic cells seem to strengthen the intestinal barrier, which affects intestinal permeability. These are just a few examples of the immune action of bacteria: thus, the mechanisms of influence of the gut microbiome are broad and not one-size-fits-all.
Objectives
The central purpose of this study is to critically examine the diversity of effects of the gut microbiome on body health. This requires a qualitative literature search and the identification of key findings from it.
Methods
The methodological basis for this study was the literature review approach, in which scientific papers on a topic are collected and analyzed in order to draw final conclusions. Digital literature databases, whether Web of Science, Google Scholar, or PubMed, were chosen for this work. Keywords such as “gut microbiome,” “microbiome immunity,” and “gut and immunity” were used to search for thematically related studies. However, additional inclusion criteria were used to narrow down the results, including mandatory writing in English, year of publication no earlier than 2018, availability of a full-text file, and authors’ credibility of the article. The latter was checked using a quick search for other papers by the same author: it was important that all articles were united by a common field of study and the discipline within which the work was written. Accordingly, any papers that did not meet these criteria were excluded from the literature search. The ethics of this study is consistent with the principles of academic integrity and states that no work has been illegally utilized or plagiarized.
Results
Of primary interest was measuring the general interest of the academic community in studying the question of the impact of the gut microbiome on individual health. Web of Science tools were used for this purpose: Figure 1 shows general chronological trends in the publication of papers on this topic. It can clearly be seen that the number of published and cited papers has increased exponentially since 2008, while previously, this topic did not seem to interest scientists at all. Interestingly, in print publications which includes not only scientific sources, the intestinal microbiome first overtook intestinal microflora in the number of mentions only in 2012, after which it never relinquished the lead. Reference to Figure 3 makes it clear that researchers from a variety of academic disciplines are interested in the gut microbiome and its relationship to health.
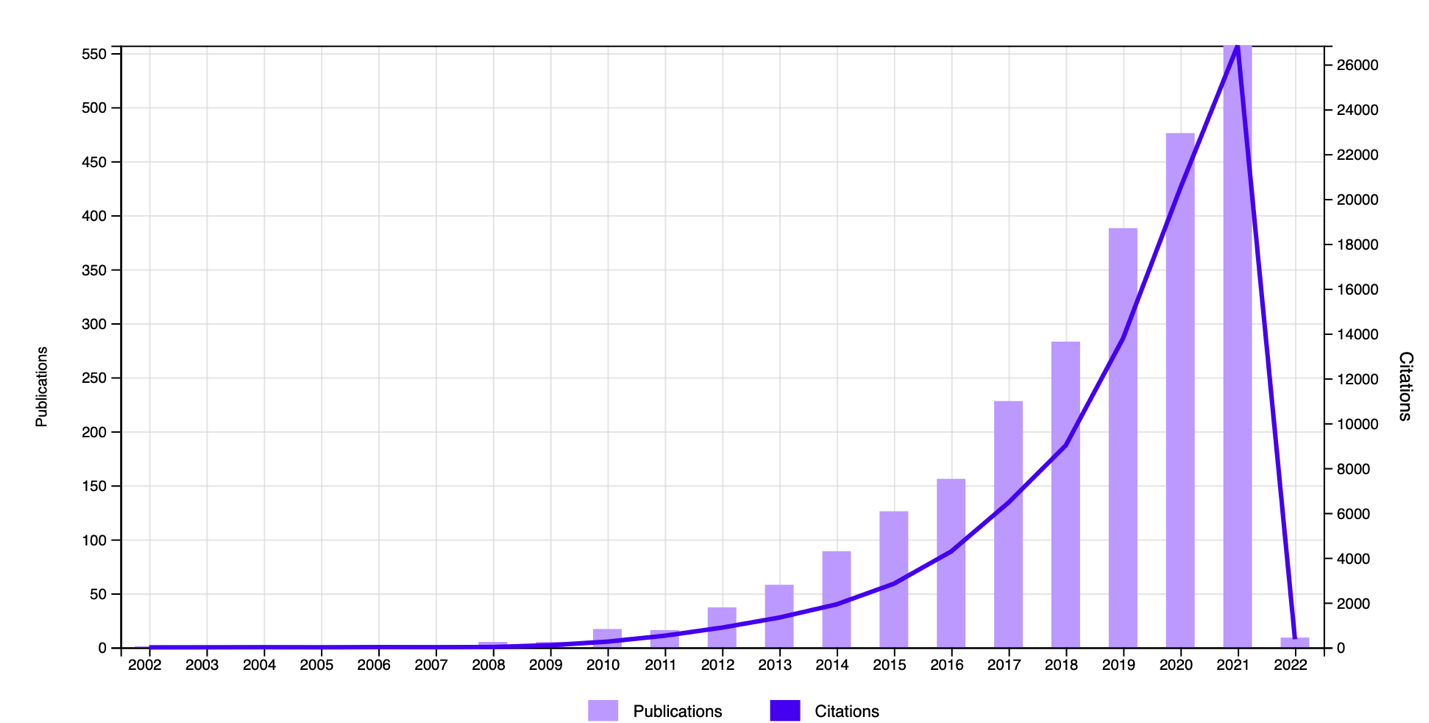
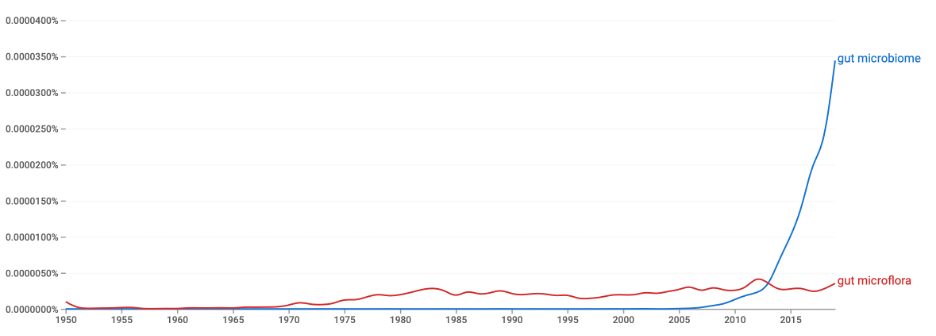
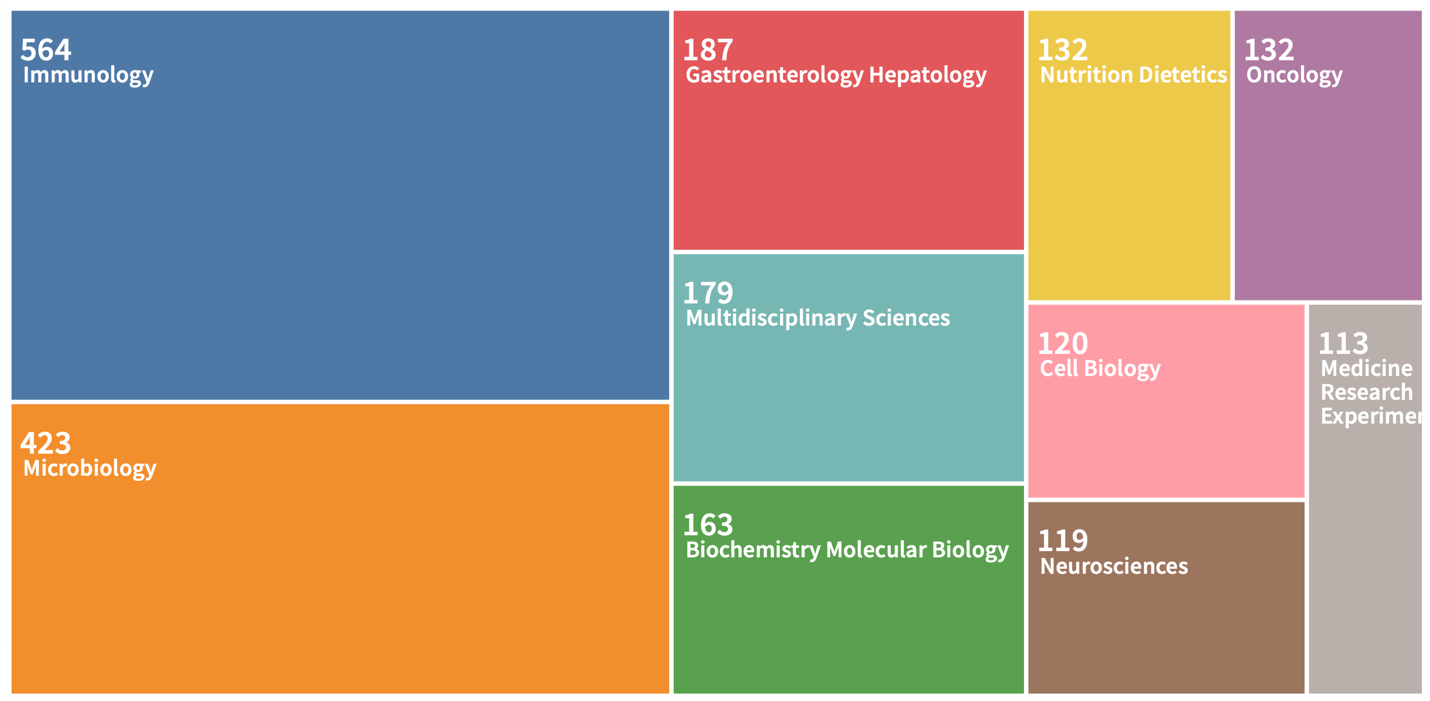
A literature search using digital platforms was very successful and resulted in a primary set of more than 50 papers. Passing a secondary filter for the uniqueness of the published conclusions left only eight research articles and dissertations, which were analyzed in further stages. This yielded a unique set of primary data that did not refer to each other.
Discussion
The present study sought to identify critical effects of the gut microbiome that directly or indirectly affect human health. A key conclusion that can be drawn from the literature search is that the gut microbiome (microflora) does not have an unambiguous mechanism for determining immunity but instead acts through a cascade of diverse reactions. For example, the gut microbiome has been shown to be extremely sensitive to change, and even neurodegenerative pathologies can lead to prokaryotic imbalance (Cani, 2018). Given the high prevalence of a variety of chronic diseases in patients, it is proper to acknowledge that studies often report different qualitative compositions of the natural microbiome. Moreover, the authors point out that the loss of microbial diversity — especially those strains that form the body’s immune tolerance — leads to the appearance of metabolic disorders, among them multiple sclerosis, obesity, and even autism spectrum disorders (Durack & Lynch, 2019). There is also strong evidence that gut-microbe-central nervous system level communication occurs through at least three channels, among them endocrine, immune, and neural connections (Martin et al., 2018). Thus, as far as the gut microbiome is concerned, it is known for a fact that it is a very sensitive system that responds explicitly to any deviation in the body and causes a chain of biochemical reactions.
Many authors of published works agree that one of the key ways in which gut microbes affect immunity is through the synthesis of short-chain fatty acids. The formation of these acids is traditionally attributed to the processing of dietary fiber by bacteria, which is then sorbed by the portal vein into the liver, entering the bloodstream from the digestive system. Usually, these short-chain fatty acids help expel pathogens, form the intestinal epithelial barrier, and initiate mucus secretion by the goblet cell. However, an equally important function of these acids is to participate in the synthesis of regulatory t-suppressor cells. These cells, in turn, synthesize the transcription factor FOXP3, which is responsible for T cell differentiation and cytokines (Di Gangi et al., 2020). This helps regulate the anti-inflammatory functions of dendritic cells, altering the intensity of the immune defense. Thus, through the mediation of short-chain fatty acids, the gut microbiome regulates the body’s immune response to pathogens or to itself.
The relationship between the gut microbiome and health is built on the principle of mutual causation, which means that each variable tends to influence the other. Thus, any pathologies and infections of the body not associated with the gut can affect the balance of gastrointestinal tract microorganisms. Conversely, altering this balance leads, as already noted, to inflammatory processes and dysfunction. This causal relationship has the critical disadvantage of artificially inhibiting the gut microbiome with drugs. Specifically, the use of drug treatments disrupts the composition and function of the microbiome, which provokes the development of intestinal infections (Weersma et al., 2020). This applies not only to antibiotic drugs but also to nonantibiotic drugs, including metformin, SSRIs, and even laxatives.
The above findings have important implications for the community because they hint at the importance of the careful selection of a thorough diet and general hygiene to keep the gut microbiome in balance. The occurrence of any food poisoning or infections in the body provokes dysbiosis, which is not always reflected as acute abdominal pain but can lead to cascading pathologies of other organs. Indeed, there is a lack of knowledge about the relationship between critical epidemic conditions, whether HIV or cancer and the gut microbiome. Therefore, the findings point to the need for more thorough and extensive research, as numerous gaps and discrepancies have been found. Additionally, there is also a need for quality public education about the impact of their gut microbiome on their health, as it is expected that for many citizens, this is not a clear connection. From a long-term perspective, these results point to the need for embryonic studies of the microbiome, as it is in the embryonic state that forms the quality composition of the human gut (Ferretti et al., 2018). On the other hand, pregnant women need to be more attentive to the development of the fetus and follow a strict diet so as not to provoke pathological changes in the prokaryotic composition of the infant’s gut. Consequently, through diagnostic procedures, it becomes possible to detect future pathologies in order to initiate timely treatment, if possible.
Keywords: Gut, Microbiome, Bacteria, Immunity, Pathologies.
References
Cani, P. D. (2018). Human gut microbiome: Hopes, threats and promises. Gut, 67(9), 1716-1725. Web.
Di Gangi, A., Di Cicco, M. E., Comberiati, P., & Peroni, D. G. (2020). Go with your gut: the shaping of T-cell response by gut microbiota in allergic asthma. Frontiers in Immunology, 11, 1485. Web.
Durack, J., & Lynch, S. V. (2019). The gut microbiome: relationships with disease and opportunities for therapy. Journal of Experimental Medicine, 216(1), 20-40. Web.
Ferretti, P., Pasolli, E., Tett, A., Asnicar, F., Gorfer, V., Fedi, S.,… & Segata, N. (2018). Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host & Microbe, 24(1), 133-145. Web.
Martin, C. R., Osadchiy, V., Kalani, A., & Mayer, E. A. (2018). The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology, 6(2), 133-148. Web.
Ruohtula, T., de Goffau, M. C., Nieminen, J. K., Honkanen, J., Siljander, H., Hämäläinen, A. M., & Vaarala, O. (2019). Maturation of gut microbiota and circulating regulatory T cells and development of IgE sensitization in early life. Frontiers in Immunology, 10, 1-12. Web.
Weersma, R. K., Zhernakova, A., & Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut, 69(8), 1510-1519. Web.
Zhang, Z., Liu, L., Liu, C., Sun, Y., & Zhang, D. (2021). New aspects of microbial vitamin K2 production by expanding the product spectrum. Microbial Cell Factories, 20(1), 1-12. Web.
Why does the Gut Affect your Health?
One of scientists’ findings in recent years is the recognition of the influence of the human gut on health. Our intestinal system is a highly complex ecosystem densely populated with bacteria and viruses. This is perfectly normal for the body as long as the balance of commensal microbes is maintained. However, any external fluctuations lead to a response from the inside, which affects the balance of intestinal microorganisms. As a result, the person gets sick.
The study of the relationship between the gut’s bacterial composition and human health has become of particular concern to scientists over the past decade. Authors worldwide are actively studying the mechanisms that cause an imbalance of gut microbes to cause inflammation. In particular, many of them attribute this to an indirect natural function of microbes to influence T-cell synthesis. Usually, T cells control the strength of the immune response. It works like a lever: T cells weaken the body’s immune defense when necessary. This is necessary, for example, to prevent the body from destroying itself from within: such pathologies are called autoimmune.
True, it should be recognized that the mechanisms of action of the intestinal microbiome on immunity are much more than mediated through T-cells. The variety of pathways also leads to different manifestations of immune failure. For example, strong evidence shows that imbalances can cause obesity, multiple sclerosis, and even autism. Consequently, the impact of the gut on a patient’s overall physical and psychological health is much broader than previously thought,
It is also interesting that the microbiome is formed in the embryonic stage, which means that the infant receives almost the entire gut microbes from the mother. That is why pregnant and nursing mothers need to follow a strict diet. Any bad habits and toxins will lead to an imbalance, which will affect the pathology of the baby. This also applies to adults: food poisoning leads to consequences much more severe than acute abdominal pain.