Introduction
One of the critical metabolic processes in the simplest living organisms is the process of energy generation. Such processes should be broadly understood as processes of cleavage of an organic substrate with the help of specialized natural molecules called enzymes. Enzymatic reactions are usually exothermic, that is, they occur with the release of energy in the form of heat (Vasickaninová et al., 2020). Part of this energy is used by organisms to maintain functional life activity, which means that it is fair to point out that these processes are key reactions for energy generation in the organism. The classification of such processes depends on the location and nature of the mechanisms involved, but the central part of the energy-producing processes in the organism is represented by cellular respiration. In cellular respiration, an organic substrate — usually glucose — is broken down to produce ATP energy. One of the landmark processes of cellular respiration is fermentation: remarkably, fermentation is not used by the human body due to the low efficiency of this biological process. Nevertheless, humans long ago learned to use the fermentation of microorganisms for economic purposes, which leads to the synthesis of desired products. A byproduct, in this case, is often carbon dioxide, which is formed by the oxidation of an organic substrate. Alcoholic fermentation is a subspecies of fermentation reactions in which glucose, which has entered the fermentation process, is broken down into ethyl alcohol and carbon dioxide molecules, as well as heat energy. Energy is released because the multiple bonds in a complex organic molecule are broken down, and the resulting bonds in ethanol and carbon dioxide require much less energy; as a consequence, the excess is released as heat. Yeast is representative of single-celled microorganisms, fungus, that use fermentation to generate energy.
Discussion
The principles described above were the basis for this laboratory work. Specifically, yeast was used as a living microorganism that could synthesize carbon dioxide as a byproduct of its alcoholic fermentation. It was this released gas that was used as a measure of the intensity of the ongoing enzymatic reaction. An initial volume strategy was used to measure directly. Specifically, water was used by the yeast as a media-forming agent and consumed during the enzymatic reaction. In other words, the amount of water decreased over time in proportion to the increase in carbon dioxide volume as the tubes were plugged.
Corn syrup was used as a substrate, a source of carbon for yeast metabolism. Corn is known to contain substantial amounts of starch, which, in turn, is broken down into glucose and fructose monomers during initial enzymatic reactions (Brennan, 2020). It follows that the intensity of the enzymatic reaction is conditioned by both the amount of substrate and the amount of yeast, which can vary in order to study the patterns. The focus of this experiment was the manipulation of the yeast quantity (concentration), which was to influence the intensity of the reaction. In other words, it was expected that an increase in the amount of yeast would entail a natural increase in the number of metabolic processes going on, which, in turn, would have a positive effect on the amount of gas released. For this reason, the purpose of the present laboratory work was to investigate the nature of this relationship and to evaluate the hypothesis in the enzymatic reaction of yeast with changes in the number of enzymes.
Results
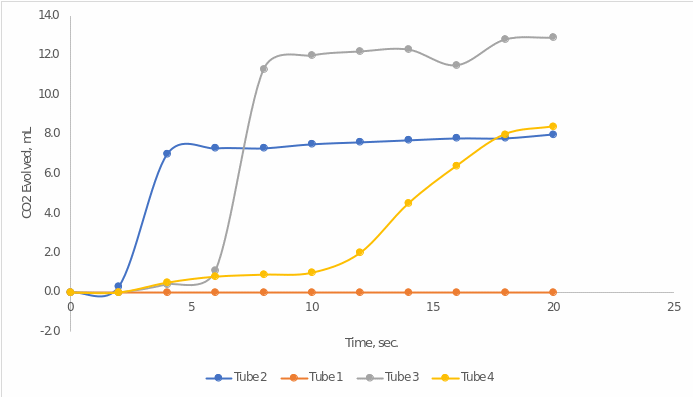
The present work sought to evaluate the effect of increasing the concentration of enzymes through increasing the number of yeasts on the intensity of the enzymatic reaction of cellular respiration. The dependent measure of this relationship was the amount of gas released. The amount of carbon dioxide released was determined by subtracting the current (decreasing) volume of water from the initial volume, as follows from the assumption of tightness of the experiment. Table 1 below shows the results of direct measurements and calculations of the emitted gas over time.
Table 1: Results of direct measurements for the four tubes
Conclusion
It is noteworthy that no changes in the context of gas production were observed in Tube 1; this is not surprising since there were no enzymes for this tube or negative control. It is for this reason that the enzymatic activity curve for this tube (Figure 1) was horizontally linear: no gas release was observed. Meanwhile, much more intriguing results were shown for the other three tubes in which the yeast amounts differed. In the second and third tubes, the amount of yeast suspension added was the same (3 mL each), whereas the last tube had 9 mL of yeast. These differences are also evident visually: the increase in carbon dioxide volume was more consistent and slower than for the other two tubes, for which a sharp increase was noticed.
References
Brennan, D. (2020). Foods high in starch. WebMD. Web.
Vasickaninová, A., Bakošová, M., Oravec, J., & Horváthová, M. (2020). Efficient fuzzy control of a biochemical reactor. Chemical Engineering Transactions, 81, 85-90.