Objectives
- To show that the time dependence of temperature is exponential
- To verify Newton’s law of cooling using a simple water cooling experiment
- To employ Newton’s law of cooling to determine temperature of water at any given time
Theory
Newton’s law of cooling according Shealy that states that the hotter an object is, the faster it cools. More precisely, the rate of cooling is proportional to the temperature difference between an object and its surroundings (1). This law is vital when examining water behavior particularly when you heat water. This is because from it, we are in a position to show how fast hot water will cool and therefore necessary adjustments can be made when doing piping. The fundamental expression for Newton’s law of cooling is:
T (t) = TA + (TH-TA) e-kt………. (1)
Where T (t) is the temperature at time t, t is time, TA is ambient temperature (temperature of surroundings), k = positive constant and TH is Temperature of hot object at time 0(Shealy 1).
Newton’s law of cooling stands for an exponential time dependence of the temperature. According to Shealy, when the rate of change of some quantity is proportional to the changing quantity itself, the quantity changes in time exponentially (2). On the other hand, the equation that represents a material that receives heat as the other losses is given by the expression: α=Ak/dcm…………. (2)
Where is A is the cross sectional area of the object, k is thermal conductivity, d is the thickness of the object, m is the mass of the object and c is the specific heat capacity of the object.
Analysis
From the three experiments, when data of temperature against time is plotted, we obtain a declining graph with which agrees with a mathematical expression of:
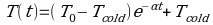 Or T (t) = TA + (TH-TA) e-k
Or T (t) = TA + (TH-TA) e-kFrom the three experiments performed, data obtained from initial temperature (To) where measured began was approximately the same within experimental error. The value for experiment A, B and C are 29.532 ±6.872E-003, 22.11 ±0.002170 and 29.634 ±0.02643oC respectively. On the other hand, from the graph, the value of Tcold for experiment A, B and C respectively, is 20.25 ±0.001984, 22.11±0.002170 and 23.08 ±0.02383 oC. Just as expected, for graph C where ice is used to cool water where the temperature difference between ice and hot water is big, we see the graph declining more up to approximately 10oC in around 2300seconds. While for experiment two or B, because of heat had to travel through the glass-Styrofoam-glass boundary, it took more time, 2500seconds to achieve stability of about 22.11oC.
Conclusion
In conclusion, from the three graphs, we see that temperature of the hot water decreased with time and an indication that the relationship between time and temperature of a hot water is exponential. For the three experiments, we also deduced that the rate of cooling between hot water and cold water depended on temperature difference and the rate of cooling decreased as temperature difference between the two became small. We see that at the start, the rate of cooling was high and declined with time. Towards the end i.e. as water was approaching stability, it took more time to see a significant temperature change. Since we have shown that relationship between time and temperature when cooling water is exponential, the objectives of the experiment were met.
Question
The cup of coffee will stay hotter longer if the cream is added immediately this is because, once the cream is added, the temperature will be decreased and therefore the conduction of heat from the cup through the walls of the cup to the table will be less. On the other hand if the cream is not added immediately, transmission of heat from the cup through the walls of the cup will be more when the temperature difference is more. From the equation
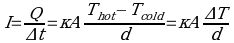 where Q is the amount gained by the cold matter and I = Q/Δt is the rate of heat flow. From this equation we see that the rate at which lost or gained is directly proportional the temperature difference between cold and hot matter. If the temperature difference is big, then the rate of heat transfer will be high and therefore the object will cool faster and in this case, a cup of coffee without cream will lose energy faster and therefore will be cooler as compared to the one where cream was added immediately.
where Q is the amount gained by the cold matter and I = Q/Δt is the rate of heat flow. From this equation we see that the rate at which lost or gained is directly proportional the temperature difference between cold and hot matter. If the temperature difference is big, then the rate of heat transfer will be high and therefore the object will cool faster and in this case, a cup of coffee without cream will lose energy faster and therefore will be cooler as compared to the one where cream was added immediately.Reference
Shealy, Malcolm. “A derivation of Newton’s law of cooling and implications for water heating.” Physics with Vernier Journa, 23.1(2010):1-10.