Introduction
A Friedel-Crafts Alkylation reaction is an eletrophilic aromatic substitution reaction that is employed in introducing tert-butyl alcohol sets on an activated benzene derivative ring. Eletrophilic aromatic substitution entails production of a positively charge tert-butyl compound as a result of the reaction between the acid catalyst and tert-butyl alcohol. After the first electrophilic aromatic substitution, the intermediate product becomes even more reactive and a second substitution occurs. In this laboratory experiment, 5ml of tert-butyl alcohol was reacted with 3g of 1, 4-dimethoxybenzene in 10ml of acetic acid as a solvent under mild heating condition until 1, 4-dimethoxybenzene totally dissolved. Cooled concentrated sulfuric acid was added drop by drop to cooled reaction Erlenmeyer flask using a non-reusable glass pipette with nonstop stirring for a period of 5 to 10 minutes. The reaction mixture was stirred for extra 5 minutes after the final drop of sulfuric acid was added. The product was then washed de-ionized water and methanol to remove impurities. The product was collected into a clean vial and reserved for melting point, TLC and IR analysis.
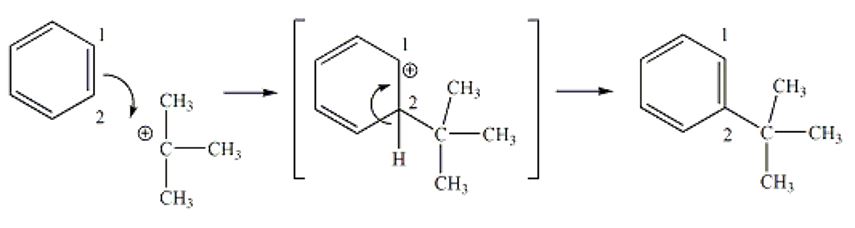
Friedel-Crafts Alkylation Overall Reaction Mechanism
A Friedel-Crafts Alkylation reaction is an eletrophilic aromatic substitution that occurs when alky group substitutes hydrogen atom on benzene derivative. Eletrophilic aromatic substitution entails production of a positively charge tert-butyl compound as a result of the reaction between the acid catalyst and tert-butyl alcohol. The pi electrons of one of the pi bonds (C1-C2) of benzene are utilized in forming a new bond between C2 and the carbon atom of the carbocation derived from tert-butyl alcohol. This leads to production of a new carbocation transitional product, where the carbocation is restricted on C1. The hydrogen at C2 gives up its electrons from the C2-H bond to regenerate the pi bond between C1 and C2, restoring the aromatic character to the benzene ring (see figure1). The same process repeats to add the second alkyl to the ring and stops due to steric obstruction, which hinders addition of more alkyl groups.
Apparatus and Reagents Used
Tert-butyl alcohol
1, 4-dimethoxybenzene
De-ionized water
Methanol
Acetic acid
Sulfuric acid
Ice for ice bath
25-mL Erlenmeyer flask
Magnetic stirrer
Hot plate
Thermometer
1 Beaker
Ring stand
Disposable glass pipette
Buchner funnel
Filter paper
Vacuum aspirator
References
Monroe, Leah. Friedel-Crafts Alkylation reaction preparation of 1, 4-Di-t-butyl-2, 5-dimethoxybenzene. 2009. Web.