Introduction
Light-emitting diode, usually called LED, is used in a variety of electrical devices They send out signals from remote controls, figure out the numbers on digital clocks, signal you when your electrical devices are turned on, and lights up watches. When put together, they can form large pictures on big screens or be used for lighting. Essentially, LEDs are just minute light bulbs that fit without difficulty into an electrical circuit.
In this paper, we will look at the simple ideologies behind these omnipresent blinkers illuminating electrical devices (The Other Power, para. 1).
Discussion
Light Emitting Diodes (LEDs) generate light in a similar way as the Neon signs. To make an LED, first, we join two conductive materials of dissimilar characteristics together. Both types of materials contain free electrons that can move about. These materials are known as semiconductors. LEDs are made of two types of semiconductors: an N-type and a P-type semiconductor, the semiconductor frequently used is aluminum-gallium-arsenide (AlGaAs). The N and P semiconductors are made by adding impurities to the semiconductor in a process known as “doping”.
N-semiconductors are negatively charged since they have additional electrons, these additional electrons move from a negatively charged region to a positively charged region. The P-type semiconductor has additional holes or “positive charges”. The holes act as pathways for the movement o0f electrons.
A diode is made using both N and P semiconductors, this set-up only permits electron flow in one direction, when zero voltage is applied, electrons move from n to P-material and form a depletion zone as shown below.
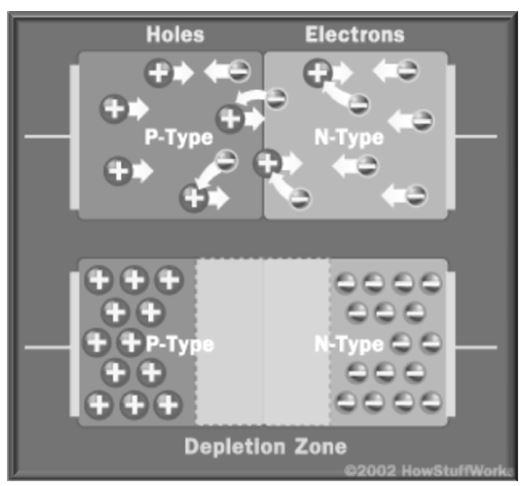
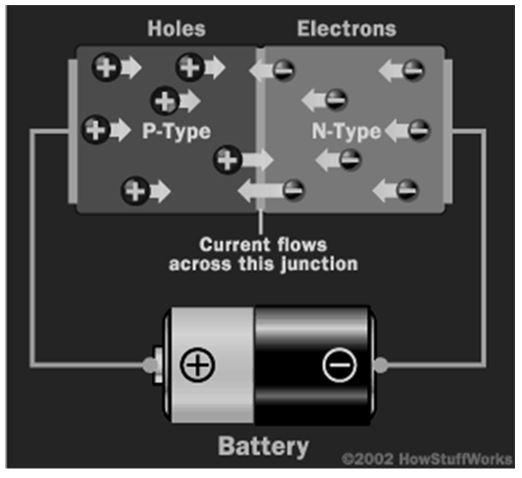
When this condition is achieved, the semiconductor regains its initial insulating state, hence there are no liberated electrons or free holes for electron motion. Therefore, the charge does not flow (charge is created when electrons flow). To resume the flow of charge, electrons must move in the reverse direction, that is, from N to P-semiconductor material. This is achieved by fixing the N-semiconductor to a negative side and P to a positive. The difference in voltage between the two sides makes it possible for electrons in the depletion zone to have free motion. Current then flows across the two semiconductors.
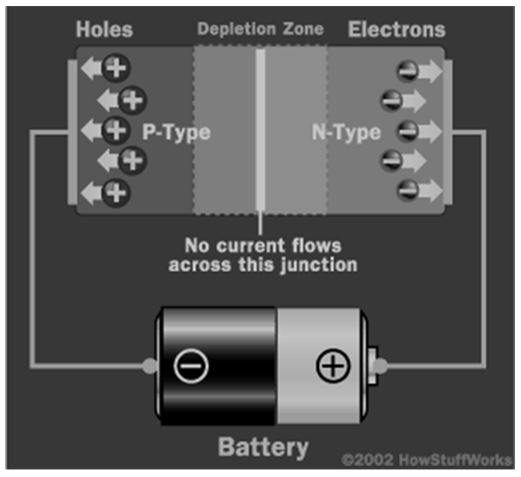
Electrons move from the P to the N-type material. The negative electrode attracts the positive holes in the P-type material. However, current does not flow through the system since electrons are moving in the opposite direction. The depletion zone increases as shown below (Mohankumar, para. 7).
The contact between electrons and “holes” in this system generates light. The light energy is released in packets known as photons, i.e. moving electrons give out photons, the most elementary division of light. In an atom, electrons move around the nucleus in a similar fashion as the planets move around the sun, the electrons’ path is known as an orbital. Electrons in dissimilar paths have varying amounts of energy: those closer to the nucleus possess the least amount of energy, electrons can also shift their orbitals (Mohankumar, para. 7).
For an electron to move to orbitals farther from the nucleus, it has to gain some energy. In a similar way, an electron gives out energy when it moves to an orbital closer to the nucleus. The amount of energy released depends on the distance between the two orbitals, this distance also affects the frequency, i.e. the higher the drop, the higher the amount energy released. Liberated electrons in a diode can drop into holes from the P-material. This constitutes a drop from a higher to a lower orbital, so the electrons release energy in bundles known as photons. This happens in all diodes, but the light energy can only be seen if the diode is made of a specific material. The atoms in a silicon diode, for instance, drop a comparatively small distance (Top Bits, 2010, para. 4). Consequently, the frequency of light energy released is so low that it is practically invisible to our eyes. Something is done to make this light visible.
Visible LEDs, such as those used for disco or party lights, are made from substances with a longer gap between the electrons’ orbitals and the nucleus. As such when they drop to lower orbitals, the photons released have a higher frequency that is visible to the human eye.
Reference list
Mohankumar, D. (2010). Light Emitting Diode How it Works?
The Other Power. (2003). Home Built LED Lighting.
Top Bits. (2010). How LED Lights Work.