Introduction
The COVID-19 pandemic has substantially impacted the world and disrupted virtually every aspect of human life. The disease is caused by severe acute respiratory syndrome (SARS-CoV-2). Investigating the molecular biology of COVID-19 is integral to creating effective strategies to combat it. The process entails understanding how it enters host cells, replicates, and avoids the immune system response.
In addition, examining the host’s reaction to infection can assist in developing treatments and preventative measures. The paper will explore the molecular biology associated with COVID-19, vaccine development, distribution, and diagnostic tests for detecting COVID-19. A comprehensive review of COVID-19 molecular properties can offer an understanding of the disease and its effect on human health.
Origins and Spread of COVID-19
Brief History of COVID-19
The first occurrences of the COVID-19 outbreak were reported to local health officials in Wuhan, China, in December 2019. Many patients showed severe respiratory symptoms, prompting an investigation by local health experts. The incident was associated with a seafood market where live animals were sold.
Healthcare professionals linked the first disease cases to animal-to-human virus transmission. The virus spread quickly within and beyond China (Groff et al., 2021). As a result, it was declared a global pandemic due to the surge of infections worldwide.
Molecular Biology of SARS-CoV-2
SARS-CoV-2 is a positive RNA virus with single-stranded RNA belonging to the Coronaviridae family. It has an approximately 125-nanometer diameter and a 30-kilobase-long genome that codes for 27 proteins, including structural, non-structural, and accessory proteins, as shown in Figure 2. The 5′-untranslated region (UTR) is necessary for viral replication, and the 3′-UTR helps with the stability and replication of the virus. Its visualization is available in Figure 1.
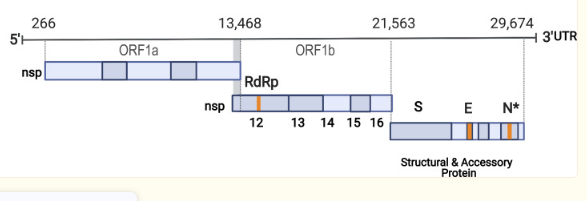
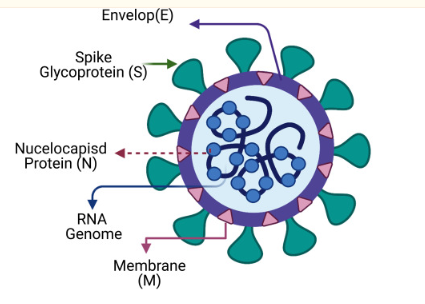
ORFs encode the viral proteins vital for viral replication and infectivity. Structural proteins include the Envelope (E), Membrane (M), Spike (S), and Nucleocapsid (N), with S being crucial for binding to the host’s angiotensin-converting enzyme 2 (ACE2) receptor. Non-structural proteins such as RNA-dependent RNA polymerase (RdRp), Papain-like protease (PLpro), and 3C-like protease (3CLpro) take part in viral replication and immune response evasion. Accessory proteins like ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 may have roles in pathogenesis and immune evasion, but their exact functions remain unknown (Alsobaie, 2021). SARS-CoV-2’s spike (S) protein allows the virus to enter host cells and has become the primary focus for vaccines or treatments against it.
Mechanisms of Transmission and Spread of the Virus
SARS-CoV-2 can be spread between different people using several methods, as shown in Figure 3. First, it mainly spreads through respiratory droplets formed when an infected person speaks, coughs, or sneezes. The droplets can be breathed in by someone close to the infected person, usually within six feet. Additionally, transmission can occur when someone touches a contaminated surface and then touches their eyes, nose, or mouth (Harrison et al., 2020).
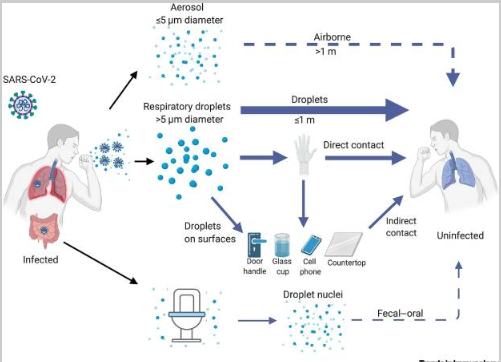
Second, aerosols, which are smaller particles that can stay in the air for longer, may be generated by activities such as singing, yelling, or working out and can potentially transmit COVID-19. Finally, it is critical to understand that SARS-CoV-2 can live on surfaces for several hours up to days, depending on the material and environmental conditions. Contact with these surfaces can lead to virus transmission if someone touches their face or the membrane linings. Furthermore, asymptomatic carriers of the virus represent a challenge in controlling the spread of SARS-CoV-2 as they could unknowingly transmit it to others (Jackson et al., 2022). The finding highlights SARS-CoV-2’s ability to colonize a patient’s throat and replicate during the early infection stage.
Molecular Mechanisms of COVID-19 Pathogenesis
The Process of Viral Entry and Infection in Host Cells
The process of how SARS-CoV-2 enters and infects host cells is complex. The first step is attachment, which entails the spike protein on the SARS-CoV-2 virus’s surface binding to the ACE2 receptor on the host cell’s surface. The spike protein comprises two subunits: S1, which contains a receptor-binding domain (RBD) that attaches to ACE2, and S2, which aids in fusion with the host cell membrane.
The next step is priming, which is facilitated by host proteases, such as cathepsins and transmembrane protease serine 2 (TMPRSS2). The proteases help cleave the spike protein into subunits S2 and S1. This process facilitates fusion by exposing the fusion peptide located in the S2 subunit. The final step entails fusion, where the viral envelope fuses with the host’s cell membrane and releases the genome into the host’s cell cytoplasm (Jackson et al., 2022). Understanding the coronavirus’s viral entry and the spike protein is essential in developing effective intervention strategies.
Virus Replication in Host Cells
When the viral RNA enters a host’s cell, it acts as a blueprint for making new viral RNA. The viral RNA polymerase is an essential enzyme that helps replicate the viral RNA by catalyzing the synthesis of different viral RNA molecules. The viral RNA polymerase may change direction during replication, resulting in a negative-sense RNA strand, which can help produce more positive-sense RNA strands. After transcribing from the viral RNA genome, the enzyme helps translate the messenger RNA into functional proteins required for replication and infection.
Accessory proteins like NSP3 and NSP5 perform complex replication and processing of polyproteins, respectively. The process is intricate, involving multiple steps, and requires hijacking the host cell machinery (Rubio-Casillas et al., 2022). Ultimately, the virus replication can result in new virus particles that can spread the infection to other cells.
The Virus’s Evasion of the Host Immune Response
SARS-CoV-2 utilizes various strategies to avoid detection by the host’s immune system and to support its replication, as shown in Figure 4. One such method is epitope masking, where the virus coats its envelope glycoproteins using glycans from the host, which prevents or minimizes antibody recognition. Additionally, the virus can decrease the expression of MHC-I molecules, which present viral antigens to CD8+ T cells, thus decreasing the presentation of such antigens and avoiding detection.
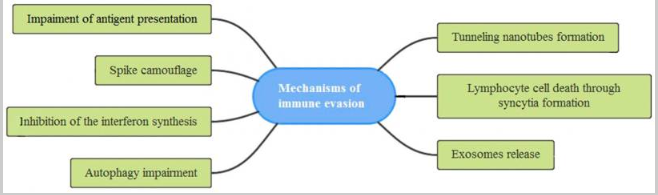
Additionally, SARS-CoV-2 interferes with interferon synthesis and may induce incomplete mitophagy, which removes damaged mitochondria from cells to utilize the host cell’s resources for replication without triggering cell death. Cytoplasmic nanotubes may also spread the virus by forming multinucleated giant cells via syncytia formation. Finally, exosomes released by SARS-CoV-2 may enable its replication while helping it evade detection by host immunity (Rubio-Casillas et al., 2022). All these mechanisms illustrate the complex interplay between SARS-CoV-2 and the host immune response and are essential for understanding COVID-19.
Host Response to SARS-CoV-2
The response to SARS-CoV-2 infection from our body is intricate and involves activating the immune system and inducing an inflammatory reaction. The innate immune response is the first layer of defense against the virus. It triggers various cells, such as macrophages, neutrophils, and Natural Killer (NK) cells, to produce substances like interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), and interferons that limit viral replication while mediating an inflammatory response.
The adaptive immune response occurs when B and T lymphocytes are activated upon identifying viral antigens. B cells generate antibodies that neutralize the virus, while T cells detect and kill infected cells. The interaction between the SARS-CoV-2 virus and the human body’s immune system determines the severity of its symptoms (Gu et al., 2022). In some cases, an overactive or weakened immune response can cause the cytokine storm, leading to a severe inflammatory reaction associated with a more severe form of COVID-19.
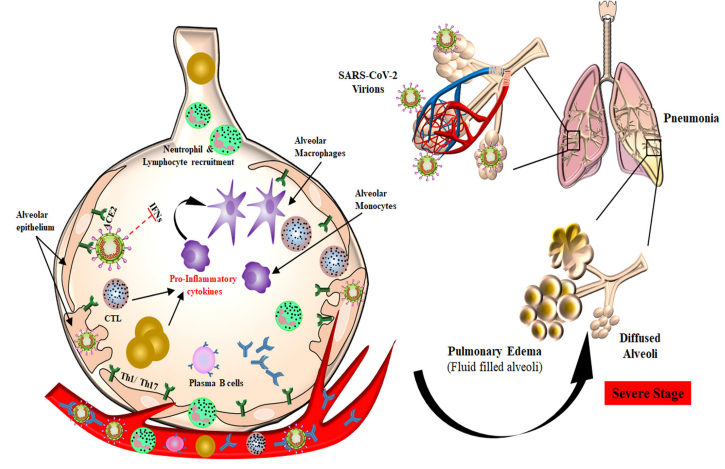
Short- and Long-Term Effects of COVID-19
The impacts of COVID-19 symptoms can vary from person to person, from mild to severe illness. Common short-term effects may include fever, cough, fatigue, loss of smell or taste, labored breathing, and sore throat. More than 500 million confirmed cases of COVID-19 and approximately 6 million deaths are currently recorded (Contreras, 2020). Severe cases of the illness can lead to hospitalization and even death (Groff et al., 2021). Overall, the short-term impacts of COVID-19 are known to healthcare experts, unlike its long-term effects.
Additionally, COVID-19 can lead to a condition known as long COVID or the disease’s long-lasting impacts. Long COVID happens when people suffer symptoms for months or years after the initial infection. The concept is poorly understood but can adversely affect one’s lifestyle and well-being. Furthermore, some people may find themselves with pulmonary fibrosis, which refers to lung damage, blood clots, or neurological problems, such as headaches and dizziness.
Furthermore, COVID-19 has been linked with mental health issues, such as anxiety, depression, and PTSD, due to the pandemic’s effects on our society. The pandemic has disrupted healthcare systems globally by delaying medical treatments and impacting mental health. Additionally, it has had a substantial economic effect, with businesses failing, often leading to job losses (Groff et al., 2021). As a result, it has exposed existing inequalities among already vulnerable groups in our society.
COVID-19 Vaccines
Several vaccines have been authorized for use worldwide to combat COVID-19, as shown in Figure 6. They include Pfizer-BioNTech, Moderna, Johnson & Johnson, AstraZeneca, and Sinovac. The vaccines have been proven to be highly effective in preventing COVID-19 infection and decreasing the severity of its symptoms. The Pfizer-BioNTech and Moderna vaccines use messenger RNA (mRNA) technology.
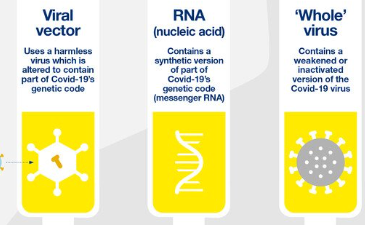
In contrast, Johnson & Johnson’s and AstraZeneca’s adenovirus vector solutions indicate they use a vector to deliver genetic material for SARS-CoV-2’s spike protein into host cells, whereas the Sinovac vaccine is inactivated. mRNA vaccines, such as Pfizer’s and Moderna’s, work by introducing a small amount of genetic material from SARS-CoV-2 into human cells, creating instructions for producing the spike protein found on its surface. The immune system then identifies the protein, producing a protective response against future infection (Mascellino et al., 2021). The adenovirus vector vaccines deliver genetic material coding for SARS-CoV-2’s spike protein directly to human cells, creating the spike protein that triggers an immune response.
The process of distributing vital COVID-19 vaccinations has been highly challenging. Certain countries and populations have experienced disparities in access to life-saving inoculations due to production constraints, logistical issues with delivery systems, and vaccine hesitancy. In some countries, governments have led immunization programs, while others have partnered with international organizations (Mascellino et al., 2021). Attempts have been made to increase available production capacity, ensure equitable access across all areas, and protect against COVID-19.
Diagnostic Tests
Diagnosis of COVID-19 can be made through various tests, such as molecular tests like the polymerase chain reaction (PCR) test and antigen tests, as depicted in Figure 7. The PCR test collects a nasopharyngeal swab from the patient, which is then processed in a lab to extract viral RNA.
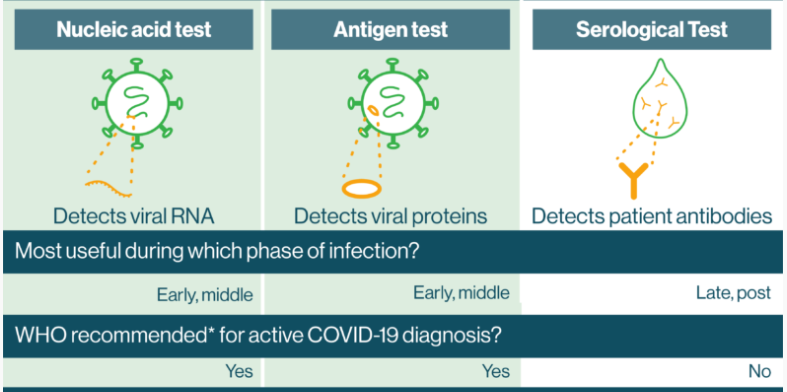
Reagents and primers specific to SARS-CoV-2 are added to the sample, then heated and cooled. If present, fluorescence-based assays will detect the amplified viral RNA (Vandenberg et al., 2021). The PCR test is accurate but requires special equipment and personnel to conduct.
Antigen tests instead detect viral proteins present in respiratory samples; antibodies are mixed with the sample and can lead to a color change if they contain proteins from the virus. These tests are faster and cheaper than PCR but are less sensitive and have higher rates of false negatives than PCR tests. Lastly, serological tests detect antibodies against COVID-19 rather than viruses (Wu et al., 2020). Specifically, they provide information about past infections or immunity, but they are not used to diagnose acute cases of COVID-19 infection.
Conclusion
A detailed analysis of COVID-19’s molecular aspects can assist in understanding its properties and impact on human health. SARS-CoV-2 is the disease’s causative agent, and it is highly infectious. Understanding the virus’s molecular mechanisms and transmission is critical to developing effective treatment and prevention. COVID-19 vaccines have been developed and distributed worldwide, offering hope of controlling the virus’s spread. Diagnostic tests, such as antigen, antibody, and PCR, effectively identify infections to prevent further transmission. Despite the success in combating COVID-19, ongoing research must focus on the disease’s long-term effects.
References
Alsobaie, S. (2021). Understanding the molecular biology of SARS-CoV-2 and the COVID-19 pandemic: A review. Infection and Drug Resistance, 14, 2259-2268. Web.
Contreras, G. W. (2020). Getting ready for the next pandemic COVID-19: Why we need to be more prepared and less scared. Journal of Emergency Management, 18(2), 87-89. Web.
Groff, D., Sun, A., Ssentongo, A. E., Ba, D. M., Parsons, N., Poudel, G. R., Lekoubou, A., Oh, J.S., Ericson, J.E., Ssentongo, P., & Chinchilli, V. M. (2021). Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Network Open, 4(10), e2128568-e2128568. Web.
Gu, W., Gan, H., Ma, Y., Xu, L., Cheng, Z. J., Li, B., Zhang, X., Jiang, W., Sun, J., Sun, B., & Hao, C. (2022). The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity. Virology Journal, 19(1), 49. Web.
Harrison, A. G., Lin, T., & Wang, P. (2020). Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends in Immunology, 41(12), 1100-1115. Web.
Jackson, C. B., Farzan, M., Chen, B., & Choe, H. (2022). Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology, 23(1), 3-20. Web.
Mascellino, M. T., Di Timoteo, F., De Angelis, M., & Oliva, A. (2021). Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy, and safety. Infection and Drug Resistance, 14, 3459-3476. Web.
Rubio-Casillas, A., Redwan, E. M., & Uversky, V. N. (2022). SARS-CoV-2: A master of immune evasion. Biomedicines, 10(6), 1339. Web.
Shah, V. K., Firmal, P., Alam, A., Ganguly, D., & Chattopadhyay, S. (2020). Overview of immune response during SARS-CoV-2 infection: Lessons from the past. Frontiers in Immunology, 11, 1949. Web.
Vandenberg, O., Martiny, D., Rochas, O., van Belkum, A., & Kozlakidis, Z. (2021). Considerations for diagnostic COVID-19 tests. Nature Reviews Microbiology, 19(3), 171-183. Web.
Wu, S. Y., Yau, H. S., Yu, M. Y., Tsang, H. F., Chan, L. W. C., Cho, W. C. S., Shing Yu, A.C., Yuen Yim, A.K., Li, M.J., Wong, Y.K.E., & Cesar Wong, S. C. (2020). The diagnostic methods in the COVID-19 pandemic, today and in the future. Expert Review of Molecular Diagnostics, 20(9), 985-993. Web.