Summary
The Emergence of a New Threat
Few clinical problems could become so urgent and relevant to the global agenda that their emergence led to radical changes in all spheres of human life. One of the main threats to the international health system remains the problem of a new coronavirus infection COVID-19 caused by the pathogenic virus SARS-CoV-2. This disease should be considered new because of the mutational phenomenon, resulting from which the pathogen was able to acquire unique and persistent evolutionary characteristics. In turn, the formation of new coronavirus varieties made it impossible for the existing healthcare systems to respond promptly to the emerging threat due to the lack of experience in dealing with this pathogen. Therefore, the emergence of SARS-CoV-2 was a significant challenge to the global agenda, demonstrating the poor performance of health systems against the emergence of new threats.
It is not only the medical industry that has been severely affected. The severe pressure on health systems has hurt virtually all areas of public life: profound causal links are readily apparent. Unable to cope with the dramatically increased flow of crisis patients, hospitals were overwhelmed and ineffective in helping to deal with the unrelenting pace of the pandemic. In turn, most governments have had to impose severe restrictive measures banning social contacts, public facilities, and significant events (Disantara, 2020). This led to the ruin of small and medium-sized businesses, forcing many commercial organizations to close and disrupt built supply chains. Moreover, to contain the growth rate of infections, many countries hastily closed borders and suspended the issuance of visas, justifying the decision on national security grounds. As can be seen, as a result of the new threat, all spheres have come under pressure, which means that public life, based on the principles of globalism, has become bruised.
Information About the Coronavirus
Coronaviruses are a well-studied group of viral particles containing RNA molecules as genetic material. The name of this family was given to viruses solely because of the similarity of their morphological structure to the solar corona. The lipoprotein envelope of the virus has several spike-like peplomers designed for efficient binding to cell receptors, as shown in Figure 1. SARS-CoV-2 is entirely consistent with this description and appears to be a naturally occurring virus, although this position still has no clear academic assessment. A central argument in the assumption of a natural origin of the virus causing COVID-19 is the high similarity rate to SARS-CoV, which caused the SARS epidemic in 2002 (Rokni et al., 2020). On the other hand, genomic studies of the structure of the virus reveal some inaccuracies and assumptions that allow opponents of the natural origin of the pathogen to discuss its laboratory production. For example, the presence of a viral laboratory at the epicenter, Wuhan, China, and the phylogenetic differences from the progenitors, and the multiplicity of mutated forms provide the basis for skepticism. At the same time, the current virus has a steady tendency to evolve through mutagenic processes of genetic material. This creates a wide variety of similar pathogens with various functionalities and makes it much more challenging to fight them effectively.
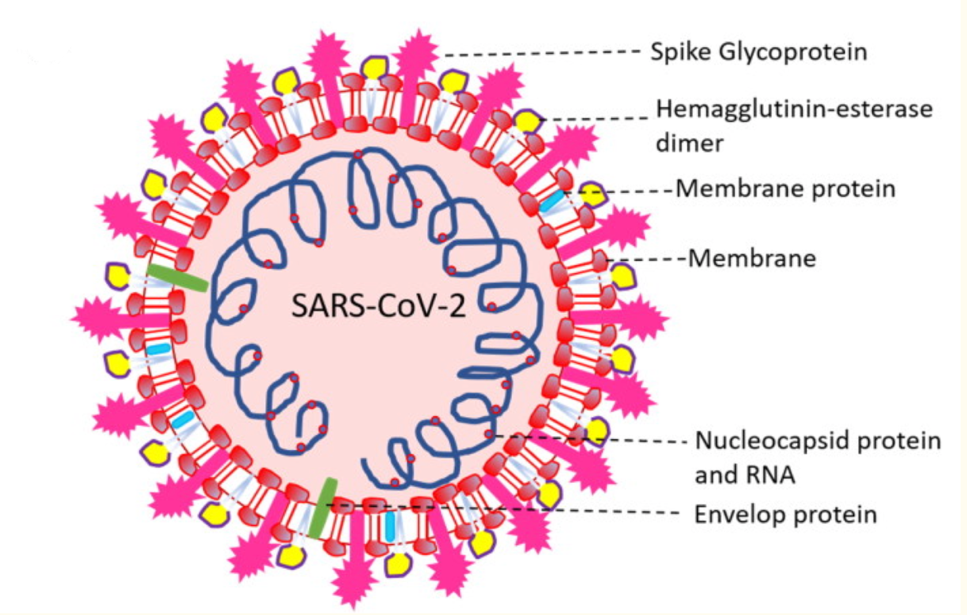
Morphological Characteristics
The morphological and genomic characteristics of the virus have already been sufficiently studied. According to Varga et al. (2020), the virions are roughly spherical or oval, with an effective diameter of about 180 nanometers. Morphological analysis of the pathogen shows the presence of multiple lipoprotein outgrowths of the viral envelope that fulfill the functional role of binding to target cell receptors and starting mechanisms to trigger infection development. Thus, each peplomer consists of a protein S complex represented by three proteins. S1 at the top of the spike interacts with the target cell receptors, while S2 and S2‘ carry out a biochemical fusion of the viral envelope with the host cell membrane to facilitate the injection of genomic material. Notably, each peplomer is generally mobile and is a hinge mechanism, which means that the attachment of SARS-CoV-2 to the cell surface proves to be more efficient for the virus. In addition to these proteins, the viral particle also has biopolymers that form the nucleocapsid envelope (Figure 1). Such proteins package the viral RNA and play a fundamental role in the assembly of the viral particle, but the structure of the coronavirus nucleocapsid is not currently described in any detail (Covián et al., 2020). From the known information, it should be noted that the internal proteins of the capsid are bound in a chain no thicker than fifteen nanometers, with the capsid as a whole not occupying the entire free volume of the pathogenic particle. Inside the nucleocapsid, as is customary for viruses, there is genomic material.
Genomic Characteristics
SARS-CoV-2 is a typical RNA-containing coronavirus with reliable morphological evidence of belonging to this taxon. The virus contains a single-stranded RNA measuring approximately 29.8 base pairs (Rokni et al., 2020). The genome itself has six open reading frames, which are characteristic of all coronaviruses and genes unique to SARS-CoV-2 responsible for synthesizing matrix and nucleocapsid proteins. SARS-CoV-2 should be assigned to the beta-coronaviruses — Coronaviridae family — among which there is SARS-CoV, which caused SARS pneumonia. All of this allows the current pathogen to be evaluated as closely related to preexisting forms but mutated and once crossed the interspecies barrier.
Aetiological Evidence
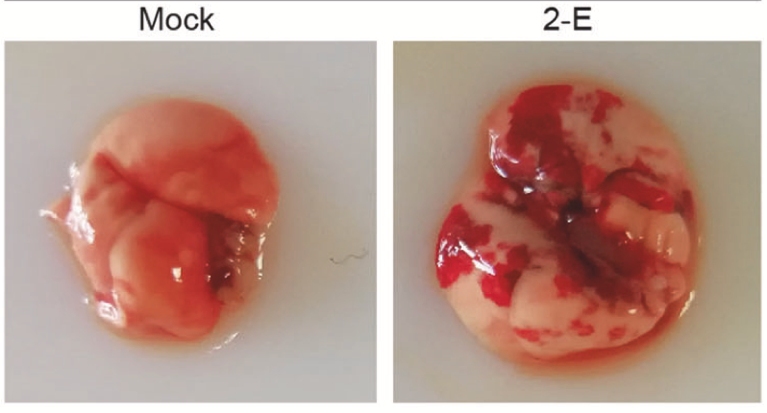
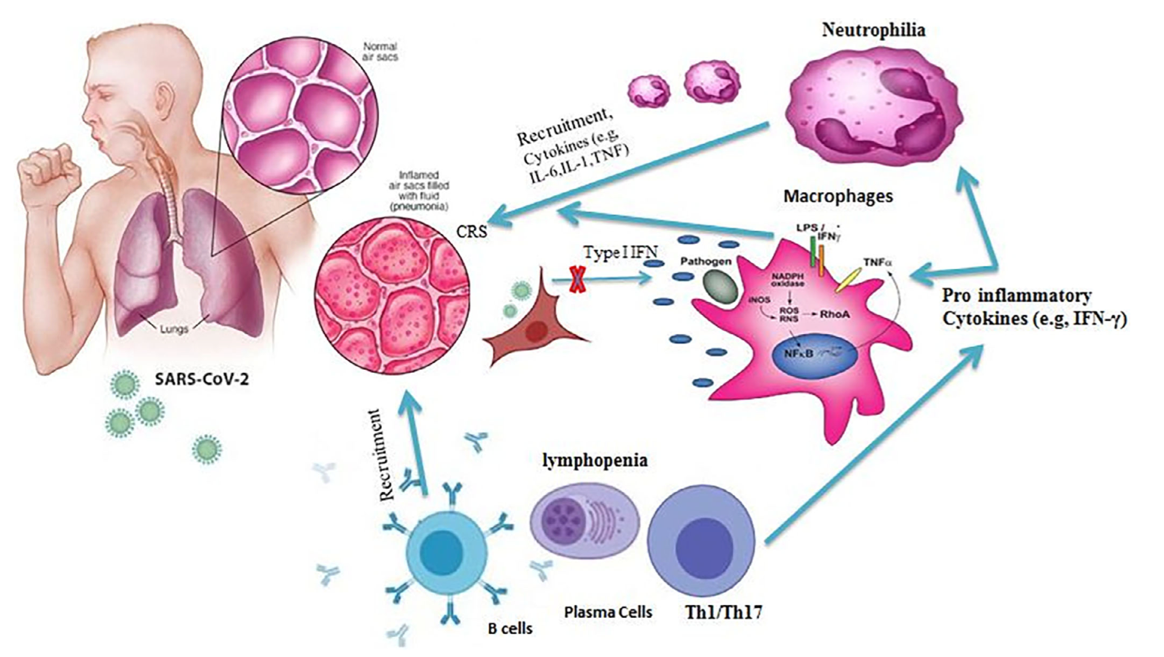
SARS-CoV-2 is a pathogen in the respiratory system of humans. The virus is capable of causing acute respiratory viral infections, including respiratory failure, as shown in Figure 2 (Xia et al., 2021). The critical entry gates for pathogen entry are the epithelium of the upper respiratory tract. Consequently, the mode of transmission of the virus involves inhaling air contaminated with viral particles, especially near the source of infection, namely a sick person or hospital. SARS-CoV-2 first attaches to target cells that have ACE2 receptors, including in the cells of the nasopharynx (Figure 3). As a result, a change in a patient’s sense of smell at an early stage of the disease may be indicative of nasopharyngeal mucosal edema. However, each of the existing developing types of SARS-CoV-2 has a unique entry into the host, so many aspects of pathogenesis need further critical study.
Vaccination as a Solution to the COVID-19 Problem
Every time humanity is confronted with a new infectious disease, the efforts of clinical laboratories rush to find an effective cure, diagnostic tests, and a vaccine. A vaccine to stimulate the formation of artificial immunity is an excellent solution, proven over time and by many generations of physicians. However, the most effective vaccine must be obtained using modern instrumental biotechnology methods and provide a targeted response to a particular virus strain. To achieve these goals, technologies have previously been developed and approved by the European and international responsible authorities for obtaining vaccine material: from attenuated, non-pathogenic pathogens to recombinant vectors carrying genetically modified copies of the virus antigens. Specifically, this study discusses the most popular forms of vaccine that have gained public acceptance worldwide: Pfizer, Moderna, AstraZeneca, and Johnson & Johnson.
Problem Statement
The emergence of the threat of SARS-CoV-2, which causes the dangerous respiratory tract infection COVID-19, has served as a significant challenge for the world. To develop a collective immunity and thus protect humanity, many laboratories worldwide have developed vaccine formulations with the potential to fight COVID-19. The vaccines discussed, Pfizer, Moderna, AstraZeneca, and Johnson & Johnson, have qualitatively different ways of biochemically protecting the body and, as a result, different efficacy.
Purpose of the Study
The purpose of this study is to conduct a detailed review of the available data revealing the biochemical features of the clinical effects of the four vaccines discussed.
Scope and Limitations
This study was performed as part of an undergraduate degree program and therefore follows high academic standards of quality. The scope of the work is determined by the breadth of the study of global health threats and vaccine efficacy and thus is characterized by considerable relevance to the agenda. Some limitations that may hinder the scaling of the findings should be emphasized. First, vaccine preparations are still undergoing clinical trials on a large sample, so it is too early to assert definitive credible data on their safety and efficacy. Second, the sources selected for this paper were initially filtered for a writing language: every one of them was written in English. There remains the possibility of ignoring potentially valuable data from those publications that have not yet been translated. Finally, only four current vaccines were studied in the paper, which means that the results obtained can hardly be approximated to the preventive drugs.
Significance of the Study
Few scientific papers to date have done a comprehensive review of several vaccines simultaneously, thus providing a comparative analysis. On the other hand, the study is characterized by high practical relevance, as the results shed light on the efficacy and safety parameters of the vaccines studied. Moreover, the source review reveals useful information about the biochemical composition of the activity of such drugs, and therefore the research work is meaningful for students, physicians, and other interested parties.
Literature Review
Among the existing clinical tools for controlling infectious diseases, vaccines should be given special attention. In contrast to classical drug treatment, vaccination is a preventive therapy to generate an immune response (Covián et al., 2020). The general pattern of forming an immune response through vaccines is the same. By injecting a foreign antigen into the patient’s body, it produces its antibodies, making it easier to recognize and capture the pathogen of future infections. Each vaccine has a targeted effect against a specific infectious agent since the mechanism of action of the drugs aims to create antibodies to specific antigens. On the other hand, vaccines differ in their methodological approaches to creation: either whole viruses or their fragments can be used as the critical protective agent. This literature review examines various aspects of the vaccination phenomenon. The review focuses on the specific vaccine products with the greatest international acceptance as a means of controlling COVID-19 infection, namely Pfizer, Moderna, AstraZeneca, and Johnson & Johnson.
Variety of Vaccines
Vaccines were thought to be defined as introducing only a dead, weakened pathogen into the human body so that the immune system could develop a preventive response to the active infectious agent. In reality, this information is outdated, as the methodology of modern vaccines has evolved considerably. Current biotechnological advances make it possible to obtain vaccines from at least two different technologies. This concerns both the injection of whole attenuated pathogen and a more innovative scheme to introduce its key fragments. Attenuated vaccines are a historical legacy of the last century, but within the current reality, the use of attenuated pathogens is often considered more expensive, unreliable, and difficult to transport and store biological material. Moreover, the technology itself for producing such a vaccine loses significantly in clinical safety, as lab technicians must have a constant supply of active viruses to obtain attenuated material. This in itself creates high pressure on the technical equipment requirement of the laboratory. The virus attenuation procedure is standardly implemented through mutation initiation, and therefore the selection of competent vectors becomes more difficult (Inagaki et al., 2021). An additional threat to the method is the inability to use the drug in immunocompromised patients, whether HIV-infected, pregnant, elderly, or children.
The immune system can create an effective protective response to a whole pathogen and its fragments. This assertion is based on a thorough understanding of the causal mechanisms that determine the formation of the immune response. For example, when a pathogen enters the body with an antigen, antibodies are formed by blood B-cells through a series of cascade reactions (Woodruff et al., 2020). There is no need to inject the whole virus, but fragments of its active parts that trigger an immune response are sufficient. For this purpose, a surface protein is used, which is produced in vast quantities using genetic engineering methods (Covián et al., 2020). Notably, low-molecular-weight proteins are rarely immunogenic, so manufacturers use antigen-carrying vectors harmless to the human body. As can be seen, this method is considered to be safer for the human body, but the genetically engineered part of it requires significant time and resources.
Genetic Characteristics of Vaccines
The action of vaccines is based on the interaction of the genetic material of the injected drug with the internal structures of the immune cells. Depending on which genetic molecule forms the basis of the vaccine, DNA or mRNA, there are two different vaccine technologies. In vector adenovirus vaccines, the drug injection contains third-party viruses that cannot replicate and are equipped with copies of SARS-CoV-2 genes. The list of such vaccines includes AstraZeneca and Johnson & Johnson: the drugs are injected intramuscularly, from where they enter the immune cells and start the triggering mechanisms of biosynthesis of the S protein of the coronavirus.
On the alternative side are mRNA vaccines, which have an RNA molecule. Vaccines such as Pfizer and Moderna deliver a ready-made instruction to target cells to synthesize a protein antigen, in response to which the human immune system produces its antibodies (Venkadapathi et al., 2021). Thus, unlike AstraZeneca and Johnson & Johnson, Pfizer and Moderna drugs do not require the use of defective viruses and thus are suitable for more immunocompromised people (Thompson et al., 2021). Consequently, mRNA vaccine production can be more extensive and adaptive: this is especially true in mutant strains. Finally, the most clear gap between the two types of vaccines used is how precisely the immune response is formed. Unlike vector adenovirus vaccines, mRNA vaccines can be administered an unlimited number of times since the patient’s immune system creates a response to the adenovirus vector, meaning that repeated administration of such a DNA vaccine would be ineffective. This leads to a necessary practical consequence: if the patient has already had an infection with this adenovirus before, the vector vaccine will not help him.
The Essence of the Two Types of Vaccines
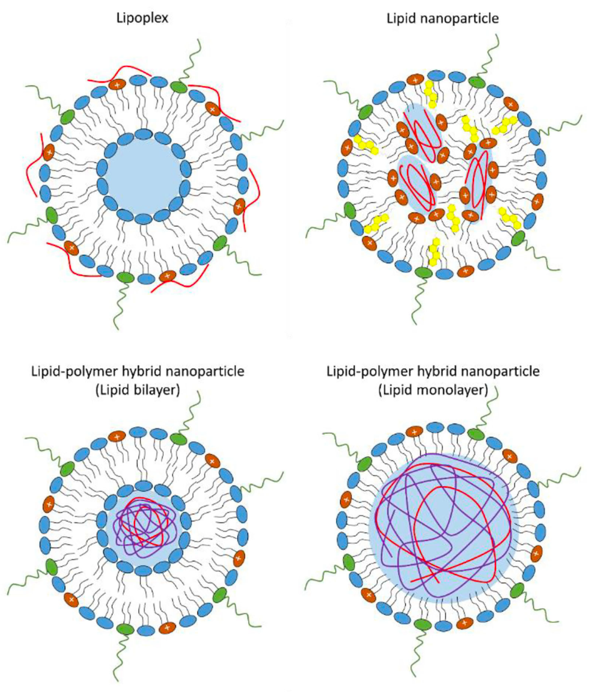
The composition of mRNA vaccines is the same since the preparations contain a lipid shell and genetic material. Pfizer and Moderna present a lipid capsule having a fragment of mRNA encoding the S-protein complex SARS-CoV-2. The point of this lipid envelope is not only to protect the internal contents from destruction but also to ease the entry of the component of Pfizer and Moderna into the cell by absorbing the lipid particle the cell (Figure 4). The mRNA vaccine will stay in the target cell in the enveloped state until the lipid capsule is destroyed due to changes in the pH of the environment. According to Guevara et al. (2020), the conditions for mRNA encapsulation must necessarily be weakly acidic to break down the fat barrier of the vaccine chemically. Once the foreign genetic material leaves the envelope, the phase of active protein biosynthesis begins: mRNA enters the endoplasmic reticulum, where protein translation occurs under the action of rRNA. Subsequently, such proteins are released outside the cell using the functions of the Golgi apparatus, after which the immune system, triggers the process of biochemical protection against the foreign protein. Concerning Pfizer and Moderna, it should be said that the immunogenic, provocative function is performed not by the added adjuvants but by the lipid membrane itself. In other words, the lipid capsule of the mRNA vaccine is modified to simplify the biochemical composition of the preparation.
The choice of the S-protein for targeting Pfizer and Moderna vaccines is not accidental. SARS-CoV-2 has been shown to use S-proteins to bind to cell receptors, and this mechanism is implemented via the RBD domain on the peplomer that recognizes the ACE2 receptor (Lainscek et al., 2020). Consequently, blocking these S-proteins by antibodies previously generated from plasma cells results in the inability of SARS-CoV-2 to actively bind to the target cell. Notably, the nature of the antibody-protein S-protein bond is based on a unique epitope-paratope interaction in which a particular antibody specifically and selectively binds to the pathogen antigen. In this context, it is essential to note that the molecular size of the S-protein is 1273 amino acids, while the size of the RBD domain does not exceed 222 amino acids (Taib et al., 2021). Considering that only a few amino acids are sufficient to connect the epitope to the paratope, it is relevant to emphasize that each S-protein has a whole host of epitopes for binding to the ACE2 receptor. As a result, the immune system must develop unique antibodies to eliminate the possibility of infection in future attacks.
In addition to next-generation vaccines, technically more sophisticated drugs such as AstraZeneca and Johnson & Johnson are gaining widespread popularity. The point of the biochemical action of such vector vaccines is to introduce into the body a DNA virus from which the E1 and E3 genes have been previously removed, and an S-protein-producing gene added instead. AstraZeneca uses the recombinant ChAdOx1 virus as the adenovirus, while Johnson & Johnson uses rAd26 to stabilize conformation (Graham et al., 2020). As a result, the adenovirus vector vaccine uses only natural mechanisms of immune system activation, so it is especially important that it cannot weaken the immune system in any way. The vector preliminarily restricts the ability to replicate, so the individual should not get sick with adenovirus infection, much less coronavirus. Unlike mRNA vaccines, vector vaccines require less stringent conditions to maintain their biological activity. AstraZeneca has been shown to use the preservative EDTA to preserve the conformational activity of proteins (Pancevski, 2021). There are opinions that EDTA can provoke thrombosis in inoculated patients, but there is insufficient reliable evidence to suggest that this is true. Occurrences of thrombosis have also been characteristic of Johnson & Johnson vaccinated patients. It has been reported that in the case of these vector vaccines, the possibility of coronavirus proteins entering the nucleus of the target cell leading to the death of the latter has not been excluded (Kowarz et al., 2021). As a result, such fragments entered the bloodstream and led to the formation of lethal clots. Notably, Pfizer and Moderna vaccines based on a different mechanism did not lead to thrombosis, which means that the problem may indeed lie in the need for the viral gene to be transcribed in the host cell.
Intermediate Efficacy of Vaccines
Most approved vaccines are still being clinically evaluated on a large sample of people, gathering information about possible side effects and adverse events. For this reason, the effectiveness of the vaccines is estimated to be relative, not finite. Pfizer was initially rated at 95% efficacy in suppressing symptomatic disease when approved by the FDA, with efficacy remaining at 90% when fully immunized (Thompson et al., 2021). Against new strains of SARS-CoV-2, Pfizer’s efficacy was only 88%. The proven efficacy of Moderna is slightly lower at 94.1%, with efficacy decreasing to 90% for complete immunization. Johnson & Johnson’s estimated efficacy is only 72% and 86% for severe diseases (Zimmer et al., 2021). Finally, the overall efficacy for the AstraZeneca vector vaccine is less than 76% and 100% for therapy of complicated cases (Vogel & Kupferschmidt, 2021). In general, without a plausibility analysis, it can be seen that mRNA vaccines appear to be more effective compared to adenoviral, which have, among others, cases of severe side effects.
As this literature review has shown, two lines of vaccines for the formation of artificial immunity against COVID-19 are currently relevant. Vector vaccines use weak viral agents that transfer the gene of interest into human cells. mRNA vaccines are more advanced forms and are represented by a lipid shell having genetic material. The proven efficacy of mRNA vaccines is comparably higher, with less overall evidence of possible adverse events for such drugs. Thus, vaccines such as Pfizer and Moderna show better performance compared to Johnson & Johnson’s and AstraZeneca.
References
Boopathi, S., Poma, A.B. and Kolandaivel, P. (2021). Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. Journal of Biomolecular Structure and Dynamics, 39(9), 3409-3418.
Covián, C., Ríos, M., Berríos-Rojas, R. V., Bueno, S. M., & Kalergis, A. M. (2020). Induction of trained immunity by recombinant vaccines. Frontiers in Immunology, 11, 1-9.
Disantara, F. P. (2020). The large scale social restrictions policy for handling the Covid-19 pandemic. Jurnal Pembaharuan Hukum, 7(2), 128-141.
Graham, S. P., McLean, R. K., Spencer, A. J., Belij-Rammerstorfer, S., Wright, D., Ulaszewska, M.,… & Lambe, T. (2020). Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 to-19. npj Vaccines, 5(1), 1-6.
Guevara, M. L., Persano, F., & Persano, S. (2020). Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Frontiers in Chemistry, 8, 963-980.
Inagaki, H., Saito, A., Kaneko, C., Sugiyama, H., Okabayashi, T., & Fujimoto, S. (2021). Rapid inactivation of SARS-CoV-2 variants by continuous and intermittent irradiation with a deep-ultraviolet light-emitting diode (DUV-LED) device. Pathogens, 10(6), 754-762.
Kowarz, E., Krutzke, L., Reis, J., Bracharz, S., Kochanek, S., & Marschalek, R. (2021). “Vaccine-Induced Covid-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Journal of Experimental & Clinical Cancer Research, XX, 1-24.
Lainscek, D., Fink, T., Forstneric, V., Hafner-Bratkovic, I., Orehek, S., Strmsek, Z.,… & Jerala, R. (2020). Immune response to vaccine candidates based on different types of nanoscaffolded RBD domain of the SARS-CoV-2 spike protein [PDF document].
Pancevski, B. (2021). A blood expert says he found why some Covid-19 vaccines trigger rare clots. WSJ.
Rokni, M., Ghasemi, V., & Tavakoli, Z. (2020). Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: Comparison with SARS and MERS. Reviews in Medical Virology, 30(3), 1-6.
Taib, S. D. M. B. M., & Alib, H. S. S. (2021). Genetic variation and evolution of 2019 novel coronavirus. Public Health Genomics, 24(1-2), 1-13.
Thompson, M. G., Burgess, J. L., Naleway, A. L., Tyner, H. L., Yoon, S. K., Meece, J.,… & Gaglani, M. (2021). Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, 2020–2021. Morbidity and Mortality Weekly Report, 70(13), 495-500.
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A.,… Moch, H. (2020). Electron microscopy of SARS-CoV-2: a challenging task–Authors’ reply. The Lancet, 395(10238), 1.
Venkadapathi, J., Govindarajan, V. K., Sekaran, S., & Venkatapathy, S. (2021). A minireview of the promising drugs and vaccines in the pipeline for the treatment of COVID-19 and current update on clinical trials. Frontiers in Molecular Biosciences, 8, 1-7.
Vogel, G., & Kupferschmidt, K. (2021). Side effect worry grows for AstraZeneca vaccine. Science, 372(6537), 14-15.
Woodruff, M. C., Ramonell, R. P., Nguyen, D. C., Cashman, K. S., Saini, A. S., Haddad, N. S.,… & Sanz, I. (2020). Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nature Immunology, 21(12), 1506-1516.
Xia, B., Shen, X., He, Y., Pan, X., Liu, F. L., Wang, Y.,… & Gao, Z. (2021). SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Research, 31(1), 1-14.
Zimmer, C., Weiland, N., & LaFraniere, S. (2021). New analyses show Johnson & Johnson’s one-dose vaccine works well. NY Times.