Paracetamol is an analgesic, originally discovered in the late 19th century. It was initially developed as a metabolite of phenacetin, a common analgesic at the time, but overlooked due to several research errors leading to its side effects being overestimated (Brune et al., 2014). It remained relatively unknown until its rediscovery in the 1940s and 1950s, when new research allowed it to be introduced to the markets as a safer alternative to contemporary pain medications (Brune et al., 2014). In quickly gained popularity, supplanting previous analgesics and antipyretics, including phenacetin.
Since then, it became one of the most widely used pain medications worldwide. It is available without prescription in most countries, and is often a first choice of pain medicine in conditions such as osteoarthritis, particularly in at-risk patients, such as pregnant women or the elderly (Brune et al., 2014). Its popularity can be attributed to its effectiveness at relieving pain, lack of side effects at recommended dosage, and low price (Brune et al., 2014). However, some researchers contest paracetamol’s safety, pointing to its possible link with acute liver failure and myocardial infarction, among other dangerous effects such as increased blood pressure or the risk of gastrointestinal ulcers (Brune et al., 2014). Due to its widespread use, paracetamol is an important drug to study, and new methods of synthesis and other reactions, seeking to clarify its potential dangers and methods of safe disposal are being researched.
Synthesis
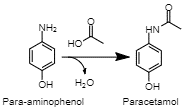
Acetylation of PAP There are several established commercial routes to synthesizing paracetamol. They primarily rely on the acetylation of a para-aminophenol (PAP) molecule (Joncour et al., 2014). However, this intermediate can be acquired in a variety of methods. (Joncour et al., 2014). In one, chlorobenzene is nitrated into 4-nitrochlorobenzene, then hydrolized under alkaline conditions, producing 4-nitrophenol (Joncour et al., 2014). Finally, this compound is hydrogenated, resulting in PAP (Joncour et al. 2014). This is one method of producing the final intermediate for the synthesis of paracetamol.
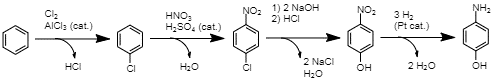
Production of PAP from chlorobenzene A second approach involves direct nitration of phenol, which is acquired from benzene through the cumene process. This results in PAP in the same amount of steps as the previous process; however, this method offers a higher atom economy of 54% (Joncour et al., 2014). However, a common drawback of these two methods is a significant production of unwanted ortho-isomers (Joncour et al., 2014). New methods of synthesis are proposed due to such drawbacks present in the methods in current use.
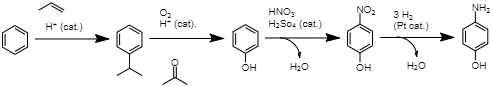
Production of PAP through direct nitration of phenol A third commercial method uses a Bamberger rearrangement to reach PAP. In this route, nitrobenzene is first hydrogenated to produce phenylhydroxylamine (Joncour et al., 2014). A Bamberger rearrangement under acidic conditions is then performed to produce PAP (Joncour et al., 2014). This method, while having a comparable atom economy, suffers from limited selectivity and production of toxic aniline as a by-product (Joncour et al., 2014). Regardless of the chosen method, the final step is transforming the PAP into paracetamol through acetylation.
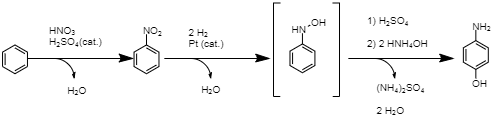
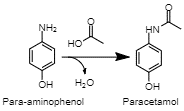
In the final reaction, PAP is acetylated with acetic acid. As this step is performed regardless of the preceding route of synthesis, production of PAP is the more differentiated part of paracetamol production. The described methods differ in substances they use, as well as selectivity and by-products. A critical feature of all three described routes is their use of sulfuric acid (H2SO4) in the production process. The use of this corrosive and polluting chemical has a negative impact on the environment, exacerbated by the large amounts of paracetamol produced (Joncour et al., 2014). This shortcoming drives modern researchers to develop new methods of synthesizing paracetamol that offer a reduced environmental impact or an improved production efficiency.
Reactions
Metabolic Toxicity: N-acetyl-p-benzoquinone imine
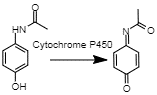
Paracetamol oxidation to NAPQI One of the by-products of paracetamol metabolism is N-acetyl-p-benzoquinone imine (NAPQI). This is a toxic compound that, although generally not produced in sufficient quantities to cause damage, can cause liver damage in prolonged use or with extant liver deficiency (Brune et al., 2014). This metabolic reaction is due to oxidation of paracetamol by the enzyme cytochrome P450 E21 (Brune et al., 2014). This reaction is critical because it lends paracetamol its toxicity and challenges its status as a relatively harmless drug (Brune et al., 2014). In the future, this can lead to a reevaluation of paracetamol’s usage.
Environmental: Oxidation by ultraviolet light and hydrogen peroxide
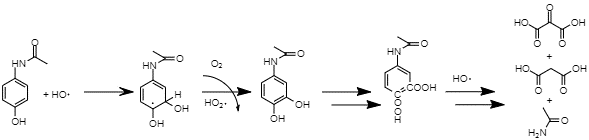
Another issue related to paracetamol is its significant potential for a negative environmental impact. As one of the most produced and used pharmaceuticals, its products, as well as the drug itself, can find its way into the water supply in significant quantities (Moctezuma et al., 2012). As such, research into the mechanism of the drug’s decay is crucial as it can inform researchers of the possible harm and help determine if specific measures are necessary to dispose of the chemical. Specifically, paracetamol oxidizes under ultraviolet light and hydrogen peroxide (H2O2).
References
Brune, K., Renner, B., & Tiegs, G. (2014). Acetaminophen/paracetamol: A history of errors, failures and false decisions. European Journal of Pain, 19(7), 953-965. Web.
Joncour, R., Duguet, N., Métay, E., Ferreira, A., & Lemaire, M. (2014). Amidation of phenol derivatives: A direct synthesis of paracetamol (acetaminophen) from hydroquinone. Green Chemistry, 16(6), 2997. Web.
Moctezuma, E., Leyva, E., Aguilar, C. A., Luna, R. A., & Montalvo, C. (2012). Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. Journal of Hazardous Materials, 243, 130-138. Web.