The Aim of the Experiment
The objectives of the experiment were to study chemical trends of elements in period 3 of the periodic table by reacting with oxygen and chlorine and consequently examining the oxides and chlorides formed.
Introduction
Chemists developed and organized the periodic table to demonstrate the recurring nature of the chemical and physical attributes of the element. Since there is a general trend of both chemical and physical properties down the group and across the period, chemists are able to predict any elements behaviour basing on their position in the periodic table. Periodic trends are a number of supplementary attributes of elements that can be associated with the element’s location the periodic table. Trends such as decrease in atomic radius, increase in ionization energy, generally decrease in reactive across the period are important behaviour that are used in predictive chemical and physical properties of elements in periodic table.
Chemical and Apparatus Used
The equipments used in the experiment included: six tubes tests, several plastic pipettes, six scoopula, test tube holder, tissue and pH test paper. The chemicals used included: NaCl3, AlCl3, MgCl2, SiO2, and water distilled.
Risk assessment/Safety
- Laboratory coat and safety goggles were worn to protect us from hazardous chemicals.
- When not in use all flames were turned to luminous to prevent accidents.
- All reactants were handled carefully as per set safety standard to avoid accidents
- Report any accident to teacher or lab technician
- Keep bench clear and tidy, wipe up small splashes with a damp cloth or paper.
Experiment procedures
Sodium, magnesium, aluminium, silicon, sulphur, argon gas and phosphorus element were put inside different seven test tubes contain chlorine gas and then heated to give respective chlorides. The formed chlorides were used to perform the next set of experiment.
Sodium chloride, magnesium chloride, phosphorus (V) chloride and aluminium chloride solid samples were put each in a separate labelled test tubes. To each test tube, distilled water was added and the solution thoroughly shaken. Results were recorded. For sulphur chloride and silicon chloride samples, due to the complexities involved, the experiment was not performed in the lab.
Using a holder, sodium, magnesium, and aluminium metals were burnt in the presence of oxygen using a Bunsen burner and results recorded. For sulphur, phosphorus, and silicon powder were put in a test tube and heated at the same time a stream of oxygen gas was directed inside the test tubes to form respective oxides. Results were recorded. The formed oxides were then put in different labelled test tubes and distilled water added to form hydroxides. Using a pH paper, the pH of each resulting solution was test and classified accordingly.
Results and Discussion
Reactions of the period 3 elements with oxygen
Most period three elements readily react with oxygen when ignited using a Bunsen burner flame. However, the reactivity rates differ from one element to another across period three with a decrease in reactivity rate as you from left to right across the periodic tablet. Sodium is the most reactive while argon being a noble gas is the least reactive due to its stable octet electronic status. Sodium burns vigorously with a golden yellow flame to give a white combination of sodium peroxide and sodium oxide. This is because the valence electron is loosely held due to weak electrostatic/electronegativity force making it easier to be kicked out from the outermost energy level (Beavon and Jarvis 47). Argon has a stable octet configuration and therefore it neither gains nor loose electrons. As a result, it does not react with oxygen. This is because as you go across the period from left to right, atomic radius decreases with increase in electronegativity force and therefore the valence electrons become more attracted towards the nucleus making it difficulty to remove it.
Based on the reaction with oxygen, the order of reactivity of period three elements beginning with the most reactive is Na>Mg>Al>Si>P>S>Cl>Ar (Beavon and Jarvis 48). This reactivity order indeed follows the ability of each individual element to lose oxygen. Below are the reaction equations between oxygen and period 3 elements under heat.
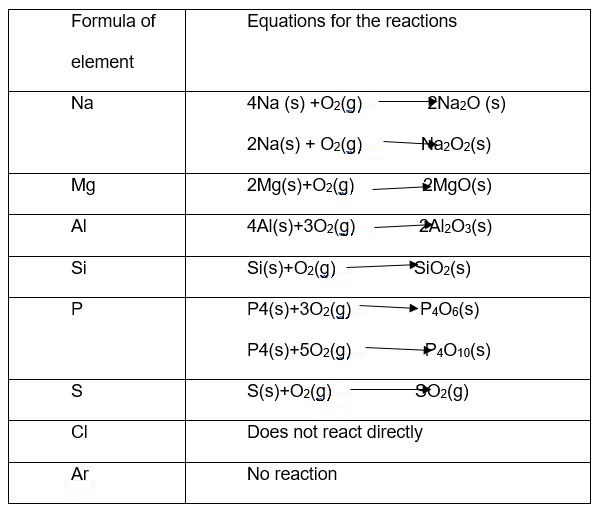
Reaction of the third row oxides
Reactions of the period 3 elements with chlorine
Most period three elements continue to burn when lowered into a gas jar of chlorine. When chlorine gas is passed over heated sodium, it reacts more readily with chlorine to form a white solid of sodium chloride while with argon gas there is no reaction. Moving across the period from left to right, the additional electron added to the outermost energy level increases electrostatic charge or electronegative force at the nucleus of the atom thereby making it more difficult for the atom to lose the valent electron (Beavon and Jarvis 49). In general, we can say that as you move from left to right across period 3 elements, the rate of reaction with chlorine decreases: for this reason, the order of reactivity of period three elements starting the most reactive is Na>Mg>Al>Si>P>S>Cl>Ar.
Below are the reaction equations between chlorine and period three elements under heat.
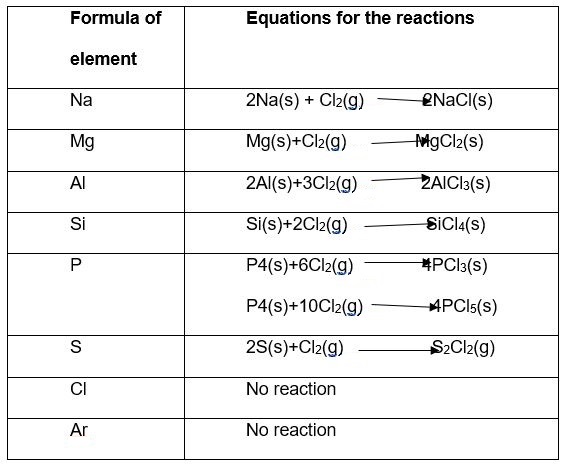
The reactions of the third row oxides
From the above table, we see that dissolving metallic oxides in water yields basic solutions with the exception of aluminum oxide, which has both acidic and basic properties (amphoteric). As you move from left to right across period 3 elements i.e. from sodium, the trend is such that the oxides move from being basic to amphoteric and then acidic. Hence, we conclude that metallic oxides such as sodium oxide and magnesium oxide which have giant ionic structures and ionic bonding tend to be basic in nature while the molecular oxides of period three elements (covalently bonded) tend to be acidic in nature (Wulfsberg 51).
Reactions of the third row chlorides
Both sodium chloride and magnesium chloride have purely ionic and contain “giant ionic lattices at room temperature” (Wulfsberg 53). On the other hand, phosphorus (V) chloride and aluminum chloride transforms their structure to covalent from ionic when they turn to liquid or gaseous.
Dissolving metal chlorides in water forms hydrated ions, which can be either basic or acidic, depend on the position of the element in the period table: sodium chloride forms a neutral solution of pH 7while magnesium chloride is a weak acidic solution of pH 6 due to less hydroxonium ions. The H3O+ ions generated by aluminium chloride in water are responsible for the acidity of aluminium chloride solution in water. however, the trend in the pH of the period three chlorides formed in water shows that the pH lowers across the period: “there is a gradual drop in pH of the chloride solutions from the metallic to non metallic “ (Wulfsberg 53). This is due to the increasing tendency of the chlorides to hydrolyse which implies that the more they hydrolyse in water, the lower their pH values and hence increasing acidity. The table below summarises the reactions of period three chlorides with water.
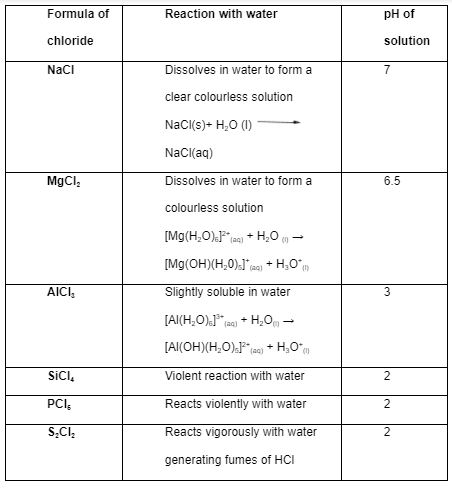
Conclusion
All period three elements except argon gas readily react with oxygen when heated to form respective oxides. Argon gas does not react with oxygen due to its stable octet electronic structure. The formed oxide readily dissolves in distilled water to form solutions of different pH i.e. sodium hydroxide was 14, magnesium hydroxide was 12, aluminium hydroxide was 8 (amphoteric), silicon hydroxide was 6 and phosphorus hydroxide being 5.
For the reaction of period three elements with chlorine gas, all element react smoothly except with argon and with itself to give respective chlorides. Since the behaviour of period three elements were successive done, the objective of the experiment was met.
References
Beavon, Rod and Jarvis, Alan. Structure, bonding and main group chemistry. Cheltenham, UK: Nelson Thomas Ltd, 2000.
Wulfsberg, Gary. Inorganic chemistry. Sausalito, CA: University Science Books, 2000.