Introduction
Polyunsaturated fatty acids (PUFAs) are important biomolecules in maintaining the health of living organisms. Therefore, they are known as essential fatty acids. However, animals cannot produce PUFAs because they lack some of the enzymes required for their biosynthesis. As a result, there is an increased interest in PUFA production in microorganisms (Elrazak, Ward & Glassey 2013). This paper describes the structure of PUFAs in bacteria, their biosynthesis, biological roles, and the antibiotic activity.
Structures of Polyunsaturated Fatty Acids in Bacteria
Fatty acids are carboxylic acids containing different numbers of carbon atoms that form a hydrocarbon chain. This chain is completed by carboxyl and methyl groups. The number of carbon atoms in fatty acid chains may range from 2 to 26 (Stoker 2015). Fatty acid chains without double bonds between two adjoining carbon atoms are known as saturated fatty acids (Naik 2015). Monounsaturated fatty acids have a single double bond, and polyunsaturated fatty acids have two or more double bonds (Stoker 2015). PUFAs can be described by a system that encompasses the total number of carbon atoms, the number of double bonds, and the position of the initial double bond in the chain from the methyl functional group at the end of the chain, which is referred to as the ‘n’ or ‘ω’ carbon (Hayashi et al. 2016).
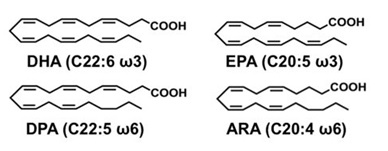
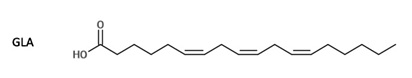
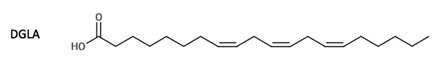
Referring to the structure of PUFAs, it is important to note that docosahexaenoic acid (DHA) is presented as 22:6n−3, where 22 stands for the total number of carbon atoms, 6 indicates the number of double bonds, and 3 shows that the position of the first double bond is on the 3rd carbon from the methyl group (Abedi & Sahari 2014). For eicosapentaenoic acid (EPA), the structure is indicated as 20:5n−3. For decosapentaenoic acid (DPA), the structure is C22:5ω6, and for arachidonic acid (ARA), it is 20:4ω6 (Figure 1; Hayashi et al. 2016). For dihomo-γ-linolenic acid (DGLA), it is C20:3n-6, and for γ-linolenic acid (GLA), it is C18:3n-6 (Figures 2-3; Desbois & Lawlor 2013).
Biosynthesis of Polyunsaturated Fatty Acids in Bacteria
PUFAs are typically biosynthesised in eukaryotic cells of such bacteria and microorganisms as fungi, microalgae, and yeast through the processes of desaturation and elongation. This pathway is known as an aerobic one, and it involves using such enzymes as desaturases and elongases. They are used to catalyse reactions in oleic acid (C18:1 ω9) (Hayashi et al. 2016). As a result of this process, methylene-interrupted PUFAs are synthesised. In prokaryotic bacteria, biosynthesis of PUFAs is observed only in the algae that also synthesises PUFAs as a result of several desaturation cycles of oleic acid. DHA, EPA, and ARA are also known as long-chain PUFAs (LC-PUFAs) that are synthesised in eukaryotes and bacteria (Hayashi et al. 2016). Thus, the aerobic pathway for synthesising LC-PUFAs is based on desaturation and elongation.
The anaerobic pathway is not typical of synthesising PUFAs in contrast to monoenoic fatty acids, but sometimes this pathway is observed in some prokaryotic bacteria with reference to utilising malonyl-CoA and acetyl coenzyme A (CoA) for the process. However, an alternative anaerobic pathway is used for synthesising LC-PUFAs. It is based on involving the polyketide synthase enzyme system and known as de novo synthesis (Yoshida et al. 2016). At the initiation stage of the biosynthesis of LC-PUFAs, the activation of the acyl-carrier proteins (ACPs) from their dormant forms is observed. The catalysis in the process is phosphopantetheinase (PPTase). The next step is the extension process that consists of the elongation, formation of double bonds, and the generation of the structure of the final product. The activated ACP accepts acetyl and malonyl groups from acetyl-CoA and malonyl-CoA in reactions catalysed by acyltransferase (AT) and malonyl-CoA acyltransferase (MAT) (Yoshida et al. 2016). The ending stage of the biosynthesis of LC-PUFA is the incorporation of biosynthesis outcomes into lipids.
Biological Role of Polyunsaturated Fatty Acids in Bacteria
PUFAs in membranes of bacteria’s cells play the same roles as in other organisms with the focus on guaranteeing the survival because of storing energy. According to Elrazak, Ward, and Glassey (2013, p. 1641), PUFAs are important for “modulating the architecture, dynamics, phase transition, and permeability of membranes and the cohesion of membrane-associated processes.” PUFAs guarantee the selective permeability of membranes in cells in eukaryotes, as well as their significant flexibility. Moreover, membrane-bound proteins are also regulated with the help of PUFAs.
Thus, LC-PUFAs mediate specific membrane tasks, including transport and the outflow activities of different compounds including antibiotics, and they play a part in the resilience of bacteria against oxidising agents, such as hydrogen peroxide (Fu, Yuan & Gao 2015; Yoshida et al. 2016). These characteristics are important to explain the biological role of PUFAs in the development of bacteria when low temperatures and high pressures are observed, as it is in case of Shewanella species (Elrazak, Ward & Glassey 2013). In this context, EPA ensures the structural integrity of cells by influencing the extent of hydration and promotes cell division at elevated pressures.
PUFAs participate in all functions of organisms, therefore, they contribute to the adaptation of bacterial cells to osmotic stress (Hachicho, Birnbaum & Heipieper 2017). For example, De Carvalho et al. (2014) reported that exposing Rhodococcus erythropolis cells to 7.5 % NaCl led to the production of such PUFAs as EPA, ARA and DPA within the first 30 minutes in order to address a new condition. Thus, PUFAs as essential fatty acids guarantee the normal functioning of organisms while affecting cells’ membranes.
Antibiotic Activity of Polyunsaturated Fatty Acids
LC-PUFAs have well-known antimicrobial and anti-inflammatory properties associated with preventing the growth of pathogenic microorganisms. As a result, LC-PUFAs are used in topical therapies for Gram-positive contagions. The antimicrobial properties of EPA, DHA, GLA, DGLA, 15-hydroxyeicosatrienoic acid [HETrE] and 15-hydroxyeicosapentaenoic acid were tested against Staphylococcus aureus and Propionibacterium acnes. According to the results of Desbois and Lawlor’s (2013) study, the LC-PUFAs were shown to prevent the growth of the bacteria at minimum inhibitory concentrations ranging from 32 to 1024 mg/L. The LC-PUFAs inhibited the growth of P. acnes and demonstrated bactericidal properties on S. aureus only. S. aureus cells were killed within 15 to 30 minutes following exposure to the LC-PUFAs. These findings allow for concluding that LC-PUFAs can be effectively used for addressing pathogenic microorganisms because of oleic acid and linoleic acid’s properties.
Conclusion
Bacterial cells need to cope with tough environmental conditions, such as extreme temperatures, osmotic stress, and exposure to antibiotics. PUFAs facilitate the adaptation of bacteria to these conditions. On the other hand, there has been a substantial decrease in the clinical efficiency of numerous antibiotics due to the prevalence of drug-resistant bacteria. The antibiotic properties of PUFAs increase their potential in the development of novel antibiotics.
Reference List
Abedi, E & Sahari, MA 2014, ‘Long‐chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties’, Food Science & Nutrition, vol. 2, no. 5, pp. 443-463.
De Carvalho, CC, Marques, MP, Hachicho, N & Heipieper, HJ 2014, ‘Rapid adaptation of Rhodococcus erythropolis cells to salt stress by synthesizing polyunsaturated fatty acids’, Applied Microbiology and Biotechnology, vol. 98, no. 12, pp. 5599-5606.
Desbois, AP & Lawlor, KC 2013, ‘Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus’, Marine Drugs, vol. 11, no. 11, pp. 4544-4557.
Elrazak, AA, Ward, AC & Glassey, J 2013, ‘Polyunsaturated fatty acid production by marine bacteria’, Bioprocess and Biosystems Engineering, vol. 36, no. 11, pp. 1641-1652.
Fu, H, Yuan, J & Gao, H 2015, ‘Microbial oxidative stress response: novel insights from environmental facultative anaerobic bacteria,’ Archives of Biochemistry and Biophysics, vol. 584, pp. 28-35.
Hachicho, N, Birnbaum, A & Heipieper, HJ 2017, ‘Osmotic stress in colony and planktonic cells of Pseudomonas putida mt-2 revealed significant differences in adaptive response mechanisms’, AMB Express, vol. 7, pp. 1-7.
Hayashi, S, Satoh, Y, Ujihara, T, Takata, Y & Dairi, T 2016, ‘Enhanced production of polyunsaturated fatty acids by enzyme engineering of tandem acyl carrier proteins’, Scientific Reports, vol. 6, pp. 1-10.
Naik, P 2015, Biochemistry, Jaypee Brothers Medical Publishers, New Delhi.
Stoker, SH 2015, Organic and biological chemistry, 7th edn, Cengage Learning, Boston, MA.
Yoshida, K, Hashimoto, M, Hori, R, Adachi, T, Okuyama, H, Orikasa, Y, Nagamine, T, Shimizu, S, Ueno, A & Morita, N 2016, ‘Bacterial long-chain polyunsaturated fatty acids: their biosynthetic genes, functions, and practical use’, Marine Drugs, vol. 14, no. 5, pp. 1-23.